Quick Contact

IVDR Regulation
The new European Union IVDR regulation described in Article 10(4), Technical File, is a mandatory requirement for all manufacturers applying for IVDR CE certification with any notified body. The IVDR replaced the IVDD and entered into force on May 26, 2017, with an implementation due date of May 26, 2022. All new in vitro diagnostic devices placed on the EU market, including class A non-sterile devices, must follow the new IVDR regulation. The major changes are as below:
- IVD classification based on Rule
- Requirements for clinical evidence and post-market performance follow-up
- Increased traceability of devices (UDI)
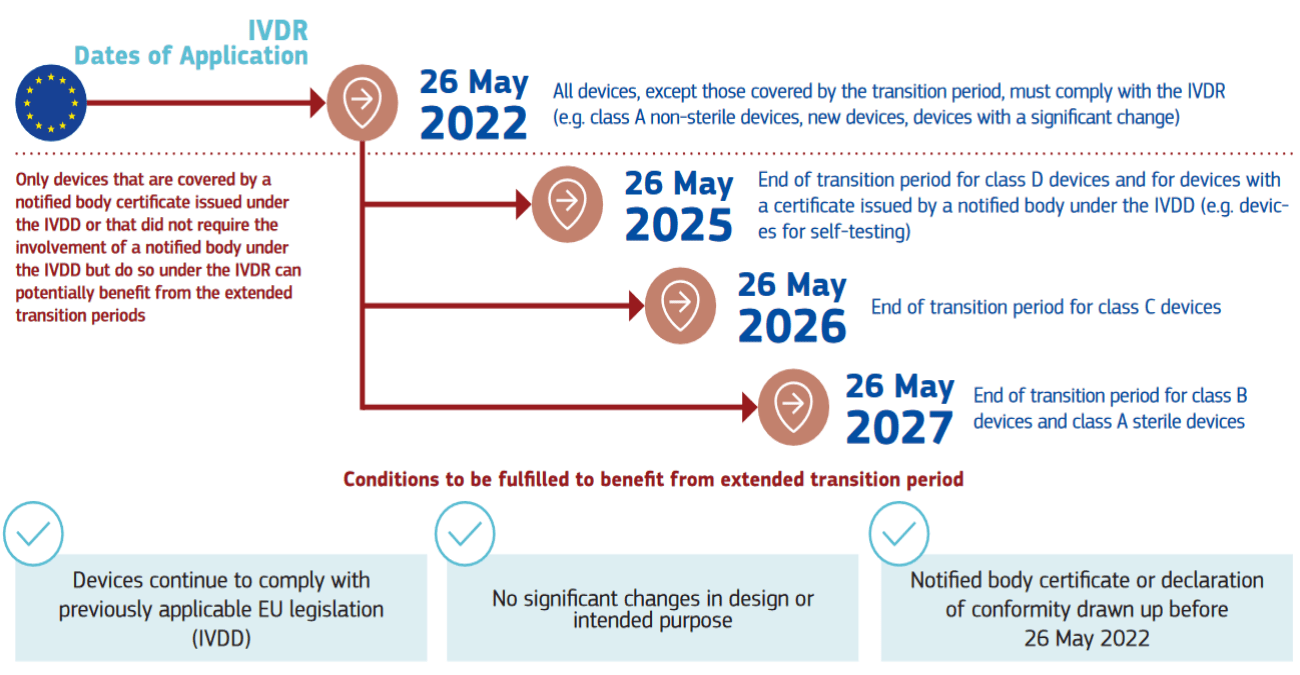
IVDR Transition Timeline
In January 2024, the European Commission released a proposal aiming to modify the IVDR 2017/746, thereby prolonging the transition periods. The primary rationale cited for this action is the shortage of notified bodies. While this doesn’t alter the IVDR’s application date, set to remain on May 26, 2022, the extended deadlines afford manufacturers and notified bodies additional time to navigate the IVDR conformity assessment process. Consequently, this ensures that safe and efficient IVD products aren’t needlessly discarded.
In-Vitro Diagnostic Device Confirmation as per IVDR
According to EU IVDR 2017/746, an in vitrodiagnosticl medical device is any medical device that is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software, or system.
Whether used alone or in combination, it is intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations derived from the human body, solely or mainly for the purpose of providing information on one or more of the following:
- regarding a physiological or pathological process or state;
- regarding congenital physical or mental impairments;
- regarding the predisposition to a medical condition or a disease;
- to establish the safety and compatibility with potential recipients;
- to forecast treatment response or reactions;
- to define or monitoring therapeutic measures.
I3CGLOBAL and its team of professionals possess the qualifications and experience to undertake risk-class IVDR projects from any corner of the globe.
Frequently Asked Questions
Is the extended timeline being applicable for all IVD manufactures? Which products are affected by the changes?
- Self-declared before May 26, 2022,
- Notified Body Certified under classes D, C, B, or A (sterile) under the IVDD
How long are the transition periods for each class?
- Class A Sterile until December 31, 2029
- Class B until December 31, 2029
- Class C devices, until December 31, 2028
- Class D devices until December 31, 2027
What requirements of the IVDR do manufacturers have to meet during the extension period?
- Post-market surveillance (PMS)
- Vigilance
- EUDAMED
- EN ISO 13485:2016
Very useful for small and medium size medical device manufactures