Quick Contact

US FDA Drug Establishment Registration
US FDA Drug Establishment Registration is a legal requirement enforced U.S. Food and Drug Administration. This process requires drug manufacturers, re-packers, re-labelers, and certain other entities involved in the production and distribution of drugs to register their establishments with the FDA. Here are several reasons why this registration is important:
Unless exempted by law, all drug manufacturers must complete the US FDA Drug Establishment Registration before they can be marketed in the USA. This is applicable for Generic, Prescription, and OTC drugs, both domestic and foreign imports.
Apply for Drug Registration with a US Agent.
Types of US FDA Drug Establishments
US FDA Drug establishments are involved in the production of drug products the U.S. Food and Drug Administration categorizes these establishments based on their specific functions. Here are the main types of drug establishments:
- New or pioneer drugs (NDAs),
- Generic copies of the new pioneer drugs (ANDAs),
- OTC drugs
- Homeopathic drugs
- Drug testing labs
- Sterilization facilities etc.
To obtain product DRUG approval, a firm must submit a New Drug Application (NDA), or an Abbreviated New Drug Application (ANDA) to the FDA. For OTC drugs, direct registration and listing are possible. Drugs sold over the counter without a prescription are called Over-the-counter (OTC) drugs. OTC drugs are classified under ‘Generally Recognized as Safe and Effective’ (GRASE) and are exempted from pre-market approval such as ANDA or NDA.
Homeopathic drugs covered in HPUSP that comply with the FDA’s Homeopathic Drug Compliance Policy Guide (CPG) also do not require FDA Approval before they are marketed. Homeopathic drugs must also be registered and listed. OTC monograph details what active ingredients may be used, at what level, and for what intended uses in the drug formulation.
US FDA Drug Establishment Registration Online
If you are planning to market drugs in the USA, it is mandatory to complete US FDA drug establishment registration. It can be completed online, but FDA won’t help you complete the registration, and it will not tell you what went wrong or where you have filled incorrect details.
An organization that submits incomplete or incorrect information cannot complete the US FDA drug establishment registration until it is corrected. Organizations or their employees who do not have prior experience in completing food facility registration, NDC requests, SPL preparation, Drug listings, annual renewal, and submitting updates find it tiresome to complete registration and related activities.
I3CGLOBAL associates and regulatory experts serving clients since the year 2000 have the required capabilities and good business sense to deal with small, medium and large-scale industries in the USA and abroad. Our services are guaranteed for success with the lowest fees!
FDA Drug Establishment Registration Process
1. Identify the drug and route of approval.
2. How Important is DUNS number?
DUNS address must match with the actual address provided for FDA registration. Deviation will lead to cancellation of registration after a few days.
3. Appoint US FDA Agent
4. NDC number request with FDA
5. SPL Preparation & Submission.
6. Update SPL, NDC Number and complete US FDA Drug Establishment Registration
7. Complete Drug Listing
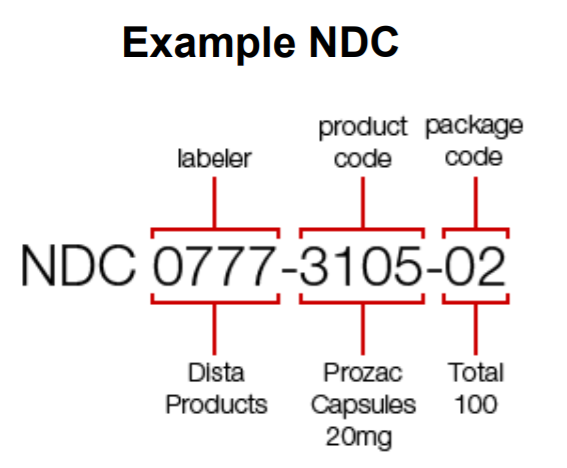
US National Drug Code (NDC Code)
The National Drug Code (NDC) is universal product identifier for human drugs in the United States by unique 10-digit, 3-segment numbering system. This is also called as NDC labeler code which is present on all non prescription (OTC) and prescription drugs.
The 3 segments of the NDC number identify the
- Labeler / Manufacturer
- Drug ,
- Commercial package size.
Frequently Asked Questions
What is the FEI Number Application?
FEI number is a unique identification number issued by the FDA to track inspections of the FDA drug facility registration. FEI numbers are also used to track GDUFA facility fee payments.
The FEI number is not for registrants, it is for FDA’s official purpose, therefore all facilities registered with the FDA are not eligible for the FEI number immediately after registration. In case any of the registered firms need, they need to request the FDA Application. Contact Us for guidance and support on this topic.
What is the role of Agent in US FDA Drug Establishment Registration & Listing?
- Act as representative on-behalf of the foreign facility
- Communicate with the FDA & Owner/Operator of the foreign facility
What is Electronic Drug Registration and Listing (E-DRLS)
Beginning June 1, 2009, FDA is accepting US FDA drug establishment registration and drug listing information electronically. FDA accepts drug establishment registration and drug listing information in XML files in SPL format.
Section 510 of the Act and 21 CFR part 207, requires establishment owner/operator in the manufacture of any formulation for use in human or veterinary drugs, to register their drug establishments and submit listing information for all drugs in commercial distribution.
Every year before December 31, the owner/operator should re-submit registration and listing information with the FDA even if no marketing activities are continued.
Who is required to SELF IDENTIFY?
- Human generic drug and active pharmaceutical ingredient manufacturers
- Finished Dosage manufacturers
- Facilities involved in packaging and labeling of drugs
- Bioequivalence study centers
What is Drug Listing? Is it necessary for all Drug Facilities to be registered with the FDA?
Listing NOT mandatory for API, Laboratories etc.