US FDA Registration Company!
US FDA Registration is mandated for every company by the United States Food and Drug Administration Department, which is responsible for monitoring and protecting public health from inferior-quality consumer products such as drugs, medical devices, food, and cosmetics.
Appointing us as an US FDA Agent can avoid conflicts when you work with multiple distributors, importers, and agents. We assure permanent US FDA compliance with regulations over the course of the period, no matter your relationship with distributors and importers in the USA.

[ultimate_info_banner banner_title=”IMPORTANT TOPICS” heading_tag=”h6″ title_color=”#0066bf” title_font_size=”desktop:24px;” title_font_style=”font-weight:bold;”]
US FDA Registration
Every company that makes a product for sale in the United States is required by law to register with the US FDA. This assures that all items sold in the United States meet a set of quality criteria. US FDA Registration benefits both consumers and companies in India and other international destinations.
Those who register with the FDA are eligible for a variety of marketing advantages that might help them boost their overall business and exports to the USA as well as other countries.
I3CGlobal and its affiliated offices in Australia, Germany, India, Malaysia, South Korea, Thailand, the USA, the UK, and Vietnam provide US FDA registration services from their respective locations. We have assisted over 8,000 companies, manufacturers and foreign exporters by registering their establishments in the United States since 2000, and we are glad to state that all of our customers have been successful in exporting to the USA without any difficulties. Our success rate is 100 percent.
US FDA Registration Process
All facilities involved in the processing of food, drugs, medical devices, and cosmetics for sale in the United States must complete US FDA Registration and Approval. The requirements are from the US Food and Drug Administration.
Failure to register will prevent you from entering the US market. All foreign facilities must name a US Agent when registering the facilities. The registration Process may feel intimidating, so we’re here to help you know exactly what you need to do to make the process go smoothly.
The FDA registration process is a demanding task that demands patience and caution while entering and submitting facility information online. It’s critical to realize that customers are not simply seeking any firm; they want to be certain that the goods you are selling are reliable and secure. The FDA wants to be certain that you are able to produce items in a GMP facility in an acceptable way with quality as a primary consideration.
The manufacturer’s information and the relevant person’s contact information must be submitted as the initial stage in the registration procedure. Additionally, you must disclose information on the manufacturing process, product specifications, product codes, regulatory number, and labeling information as necessary for the items.
Once the mandatory information is submitted along with the DUNS number, the Food and Drug Administration will issue a US FDA registration number. FDA Cosmetic Registration and and food facility registration details are not published on the FDA website, whereas medical device and drug establishment registration details will be published there.
If they approve it, then congratulations! You can move onto the planning phase for exporting goods. Remember that exported goods must comply with FDA labeling regulations.
I3CGlobal and its affiliated offices in Australia, Germany, India, Malaysia, South Korea, Thailand, the USA, the UK, and Vietnam provide US FDA registration services from their respective locations. Contact us for fast and economical service with high quality and full customer satisfaction.
How to get US FDA License?
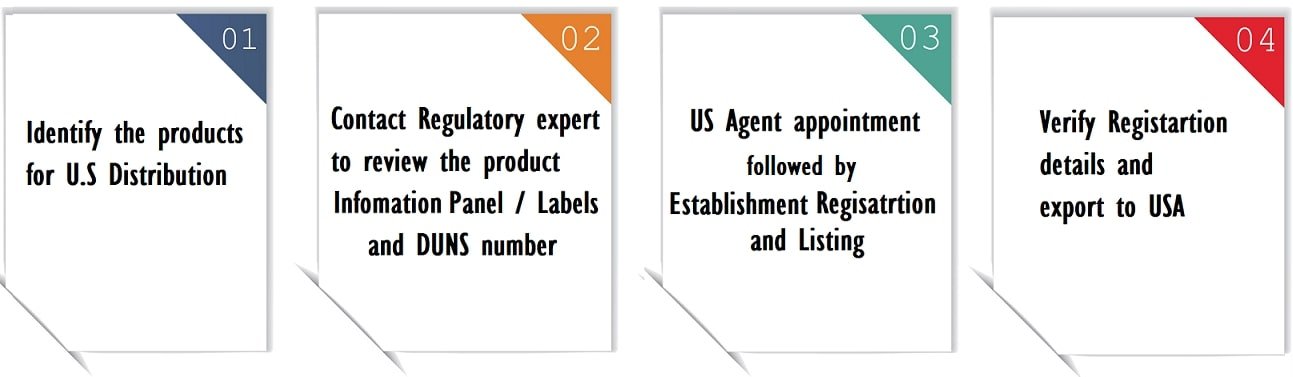
Step 2: Appoint US FDA Agent and Register the Establishment / Facility
If the product is covered under US FDA Regulations, then your company need to obtain an FDA registration number and have a listing done for the products, if applicable. Details can be submitted to an US FDA Agent for onward submission to the FDA.
Step 1: Determine the product covered in the US FDA Registration scope
The first step is to determine whether your product requires US FDA registration. You can use the FDA’s Mobile Device Software Validation Tool to find out whether your software application or device is regulated by the US FDA. Only healthcare products are covered by the FDA.
Step 3: Label Compliance and Good Manufacturing Practice (GMP)
The primary information panel of the products must be in compliance with FDA labelling guidelines specific to the product. The FDA issued separate guidance documents for cosmetics, drugs, medical devices, and drugs. We also provide a label review service.
The manufacturers, reprocessors, re-labelers, and warehousing agents must assure GMP is followed in the facility. FDA authorities can come for an inspection at any time to check the production and quality control activities.
Advantages of FDA Registration Services by I3CGlobal
Every year, hundreds of businesses in the United States are required to register their goods with the US FDA. It’s a massive endeavor, and it’s frequently a cause of uncertainty and irritation for enterprises seeking to break into a highly regulated field.
I3CGLOBAL works with the US FDA from our offices in Australia, Germany, India, Malaysia, South Korea, Thailand, the United States, the United Kingdom, and Vietnam to assist you in speeding up the process of having your facilities registered. You can relax knowing that your firm is in compliance with all applicable legislation and that you’re receiving the same high-quality service that you’d expect from a company based in your own country.
I3CGLOBAL is your one-stop shop for registering your facility and listing goods in the United States, with over twenty-two years of expertise in US FDA Registration. We’ll assist you in navigating the FDA’s complicated regulatory standards to ensure that your products are given a fair shake. We’re dedicated to providing you with exceptional customer service, and we have the reviews to prove it.
Why should hire US Agent for FDA Registration License?
In order to be able to sell products in the United States, you need a US FDA registration. This is a process that involves submitting an application and paying a fee. Every company that makes a product for sale in the USA is required by law to register with the US FDA.
The US FDA regulates many products that are intended for human consumption, including food and medical devices. In addition to registering your product with them, you will also need to comply with all other applicable laws and regulations.
This includes following good manufacturing practices (GMPs), which are guidelines for how to manufacture products safely. When you register your product with the FDA, you’re confirming that it’s been tested and found to be safe for public use. You also have to include information about how to use the product, what results you can expect from using it, and any possible side effects or risks.
Reach out to us for swift, cost-effective services that guarantee high quality and complete customer satisfaction.
Frequently Asked Questions
What is US FDA Registration number?
US FDA Registration number provided by FDA soon after acknowledging the submitted information. It’s also the confirmation of registration. The Number allocated mainly for Food Facilities and others is updated online.
How to Obtain a US FDA Certificate?
The US FDA is the government agency in charge of regulating food, drugs, and medical devices. The agency was created to ensure products are safe for public consumption.
Before bringing a drug to market, manufacturers must conduct lab, animal, and human clinical testing and submit their data to FDA. After reviewing a manufacturer’s data, FDA may decide that the product is safe for public use. If FDA deems it necessary, they will require further tests before approving the product.
If you’re looking to bring a new drug to market or sell a medical device that hasn’t been approved by FDA, reach out to us! We have years of experience getting FDA approval for products.
What is the difference between US FDA and FDA Registered?
US FDA approves only prescription drugs, research formulas, and critical medical devices only. There is no FDA-approved drug database for the public.
How much cost for FDA approval?
The cost and timeline for FDA approval depend on the product and nature of the product.
What are the benefits of US FDA Registration?
This assures that all items sold in the United States meet a set of quality criteria. Registration benefits both consumers and companies. Those who register with the U.S. Food and Drug Administration are eligible for a variety of marketing advantages that might help them boost their overall business and exports to the USA as well as other countries.
Consumer confidence: When your customers see that you’re registered with the FDA, they will trust you more because they know you’re committed to producing safe and high-quality products.
Better Brand Image: When you’re registered with the US FDA, it gives your business an air of legitimacy and increases your visibility as a trustworthy business. Your customers will know you adhere to high standards and will trust you more as a result.
Proof of Quality & Safety: The US FDA has strict policies in place to ensure product safety throughout every stage of production. This helps protect consumers from unsafe products entering markets where
When your customers see that you’re registered with the FDA, they will trust you more because they know you’re committed to producing high-quality and safe products.
How do I verify FDA certificate?
FDA will not issue an FDA certificate.
Where do I find my FDA Registration number?
- Medical Device:
- FDA Drug Establishments:
- Drug Labels:
How long FDA approval takes?
Does my product need FDA approval?
If the medical device falls under high risk, 510k approval is required or any generic /prescription drugs need approval or certain food /pharma colour additives need approval.
How to verify my facility needs FDA approval or FDA registration?
In the case of Medical Devices based on the classification and device FDA code or in the case of Drugs if not covered in the monograph you can understand whether prior approval is required or not!