Quick Contact

IVDR Regulation
The new European Union IVDR regulation described in Article 10(4), Technical File, is a mandatory requirement for all manufacturers applying for IVDR CE certification with any notified body. The IVDR replaced the IVDD and entered into force on May 26, 2017, with an implementation due date of May 26, 2022. All new in vitro diagnostic devices placed on the EU market, including class A non-sterile devices, must follow the new IVDR regulation. The major changes are as below:
- IVD classification based on Rule
- Requirements for clinical evidence and post-market performance follow-up
- Increased traceability of devices (UDI)
- Person Responsible for regulatory compliance
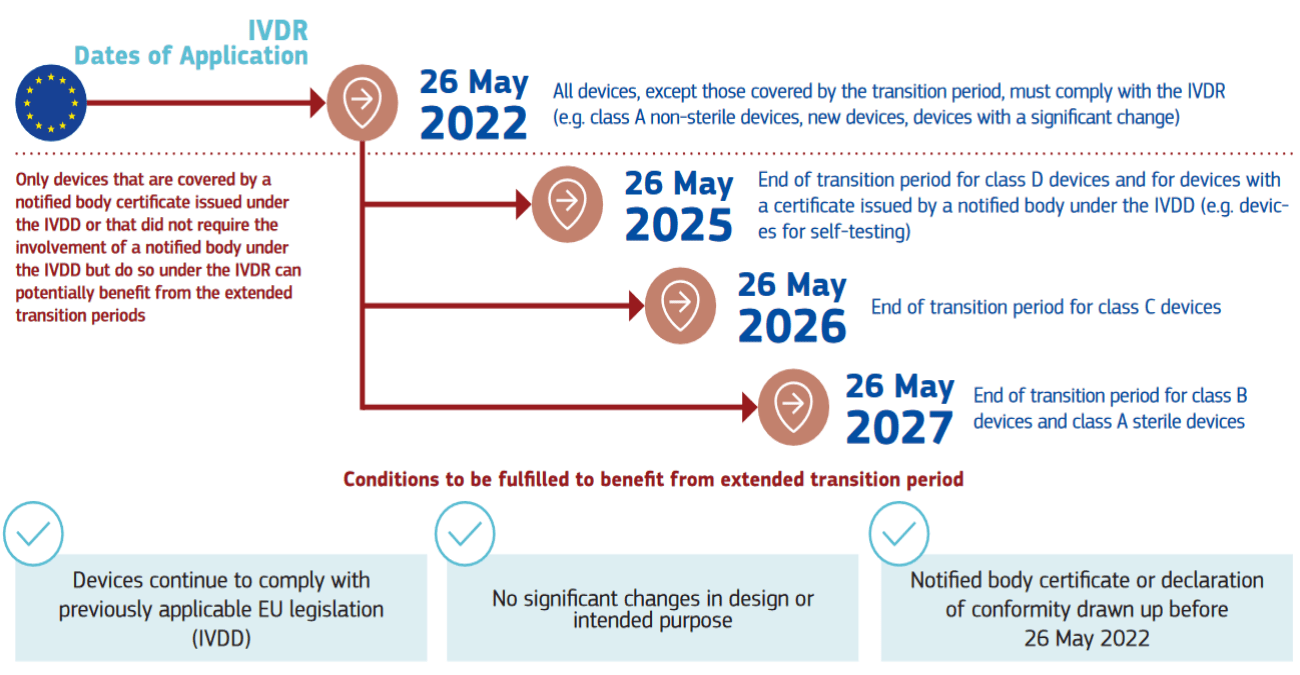
IVDR Transition Timeline
In January 2024, the European Commission released a proposal aiming to modify the IVDR 2017/746, thereby prolonging the transition periods. The primary rationale cited for this action is the shortage of notified bodies. While this doesn’t alter the IVDR’s application date, set to remain on May 26, 2022, the extended deadlines afford manufacturers and notified bodies additional time to navigate the IVDR conformity assessment process. Consequently, this ensures that safe and efficient IVD products aren’t needlessly discarded.
IVDR Regulation and Implementation Strategy
Device Identification and Planning: Start with a GAP analysis followed by identifying the project management team. This team is responsible for coordination with consultants and notified body
Technical Documentation: Conduct Performance evaluation followed by the development of a report. Transfer the data from PER to the technical documentation file. The facility should be thoroughly assessed by consultants for strict implementation of QMS. ( Make sure subject expert advice on study design and formulation to avoid any impact on specificity and sensitivity)
Notified body conformity assessment: Submit technical documentation to the notified body, respond to file review comments,
Post-market Launch: performance evaluation must be designed to be a continuous activity, involving the gathering of clinical evidence throughout the lifespan of the Invitro diagnostic device. This is achieved with the assistance of the post-market performance follow-up process. No European manufacturers must involve the service of European authorized representatives.
In-Vitro Diagnostic Device Confirmation as per IVDR
According to EU IVDR 2017/746, an in Vitro diagnostic medical device is any medical device that is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software, or system.
Whether used alone or in combination, it is intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations derived from the human body, solely or mainly to provide information on one or more of the following:
- regarding a physiological or pathological process or state;
- regarding congenital physical or mental impairments;
- regarding the predisposition to a medical condition or a disease;
- to establish the safety and compatibility with potential recipients;
- to forecast treatment response or reactions;
- to define or monitor therapeutic measures.
Team I3CGLOBAL provides strategic guidance for IVDR Regulation. We have the necessary expertise and know-how, manpower to navigate EU 2017/746 regulations for small, medium, and large-scale manufacturers globally by supporting all stages of technical documentation. Outsource the work to I3CGLOBAL and rest assured with us!
Frequently Asked Questions
Is the extended timeline being applicable for all IVD manufactures? Which products are affected by the changes?
- Self-declared before May 26, 2022,
- Notified Body Certified under classes D, C, B, or A (sterile) under the IVDD
How long are the transition periods for each class?
- Class A Sterile until December 31, 2029
- Class B until December 31, 2029
- Class C devices, until December 31, 2028
- Class D devices until December 31, 2027
What requirements of IVDR regulation manufacturers have to meet during the extension period?
- Post-market surveillance (PMS)
- Vigilance
- EUDAMED
- EN ISO 13485:2016
How important performance evaluation in IVDR Regulation?
The IVDR performance evaluation involves a combination of Scientific validity, Analytical performance, Clinical performance and Scientific peer-reviewed literature. The PER contains all the clinical evidence including the scientific validity report, analytical performance report, and clinical performance report. 2017/746 IVDR regulation more focus on PER.