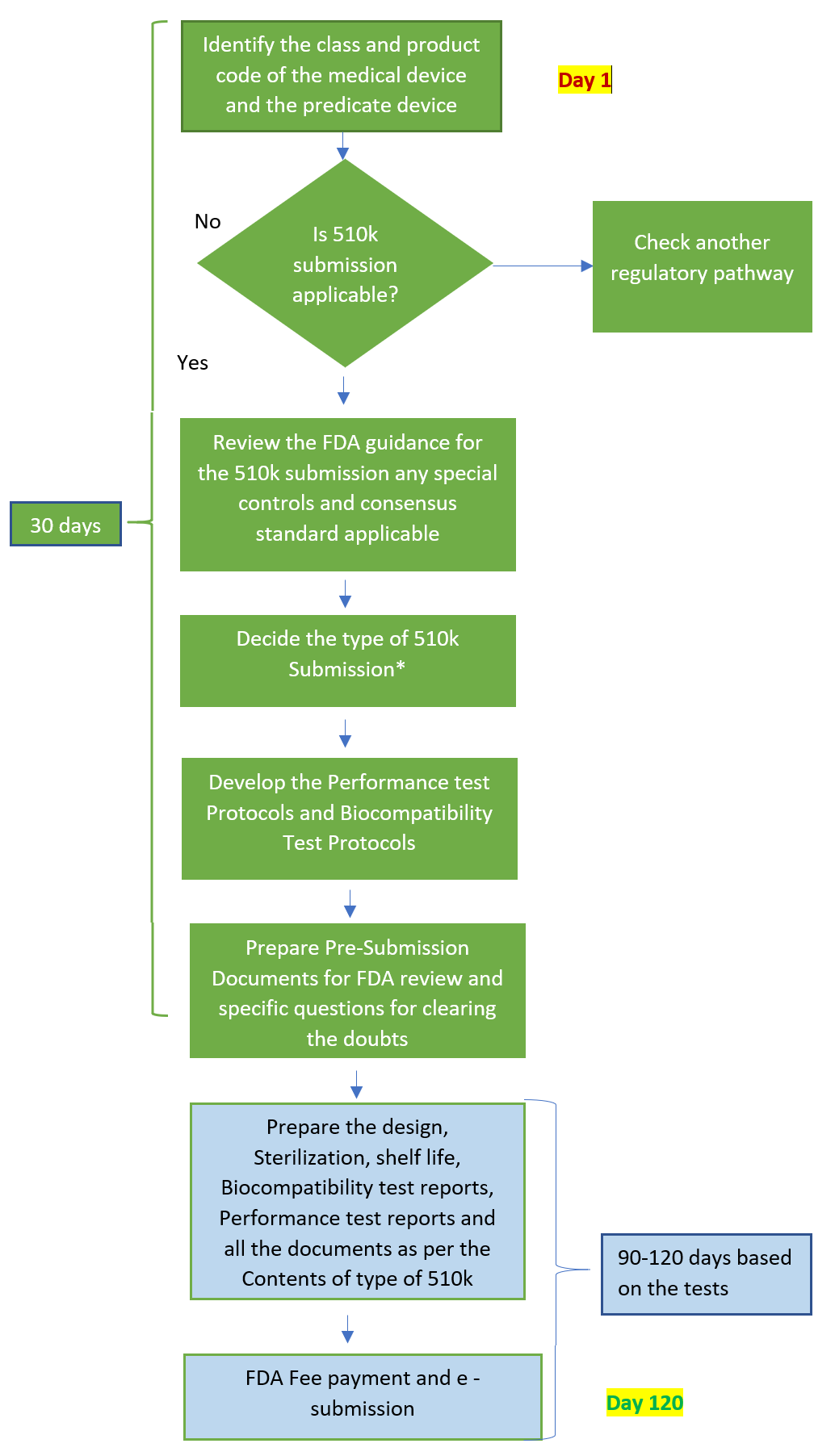
FDA 510k Submission
FDA 510k Submission is made to the food and drug administration to demonstrate that the device is substantially equivalent to a legally marketed predicate medical device that is not subject to premarket approval.
510k Clearance is required when you plan to market a device in the U.S. that is not exempt from 510k requirements and is not already legally marketed. It is also required when there is a significant change or modification to an existing device that could affect its safety or effectiveness. Preparation involves gathering all required documentation, conducting necessary testing, and ensuring that the submission meets all regulatory requirements.
510(k) Submission document preparation including device testing, and clinical data consolidation takes up almost 4 to 5 months. Post submission to the FDA generally take up to 6 months, for closing the review queries. Engaging with a regulatory consultant can help ensure a complete and compliant submission.
510k Consultants will carefully plan, make strategic decisions, and speed up the whole process without RTA or a large number of additional information requests or major review comments. All foreign manufacturers are requested to have a US Agent for FDA correspondence officially.
Note: The service of the US Agent is different from the US Agent service for FDA Establishment Registration.
Would you like us to send an email with important information within the next 2 minutes? Kindly share your email below.
Pre or Q FDA 510k Submission
The Pre or Q FDA 510k Submission program allows medical device manufacturers to obtain FDA feedback on various topics such as test selection, standards, testing protocols (Biocompatibility, safety, performance), substantial equivalence, regulatory pathway, or any other specific technical issues before submitting a formal application
An FDA Premarket Submission is typically initiated by submitting a detailed request to the FDA that outlines specific questions or concerns. The request should include a description of the device, regulatory history, specific questions for FDA feedback, and supporting information. The FDA generally schedules a meeting (teleconference or in-person) within 60 to 75 days of the request to discuss the questions and provide feedback.
Benefits of Pre Submission
Although Pre / Q submission is not mandatory, we often recommend customers do so to gain an FDA view of the documents and if you have specific questions to the FDA to clarify. The consultants use this provision to seek valuable feedback on various topics, such as costly bench and animal testing, and clinical trials.
Early feedback from FDA help improve the quality of the files and avoid potential pitfalls and ensure their FDA 510k Submission early clearance. It also expedites the overall review process by addressing critical issues before the formal submission. Pre-submission allows in aligning the requirements, reducing the risk of delays and rejections or shorter the total review times
It is important to note that the feedback provided during a Pre-Sub is non-binding, it can be a strong indicator of the FDA’s expectations, but it is very crucial to write/ask the questions and arrange supporting information to get good feedback in response.
We offer the best guidance and solutions for FDA 510k submission to worldwide manufacturers and specification developers. Our 510(k) services are time-bound and economical. We match prices. So partner with us and experience the difference firsthand.
FDA 510k Submission Timeline
The duration of the FDA 510k submission process and timeline can vary significantly depending on factors such as the complexity of the device, the completeness of the submission, and any interactions required with the FDA. Generally, the Food and Drug Administration aims to complete the review process within 90 days of acceptance, but actual timelines may be longer in the majority of the cases of applicants.
|
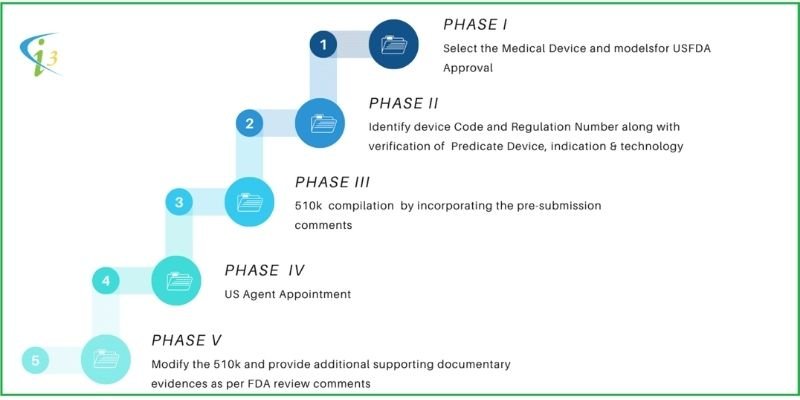
PHASE I |
Stages |
Activity |
Responsibility |
Timeline |
|
1 |
Select the Device and models for FDA 510k Submission |
CLIENT |
20 Days |
|
|
2 |
Identify Predicate Device with the same indication and technology |
CLIENT + I3C |
||
|
3 |
If NOT substantially equivalent, follow the PMA route or, if substantially equivalent, follow the 510(k) route |
CLIENT + I3C |
||
|
4 |
Appoint I3CGLOBAL as Technical Consultants and US Agent |
CLIENT |
||
|
PHASE II |
5 |
Identify Device Code and Regulation Number along with verification of Predicate Device, indication & technology. |
I3C |
90 Days |
|
6 |
Identify the device Class and guidance document |
I3C |
||
|
7 |
Biological evaluation and test requirement identification in line with the predicate device |
I 3 C |
||
|
8 |
Samples send to the Laboratory |
CLIENT |
||
|
9 |
Evaluation of equivalent device compilation |
I3C |
||
|
10 |
Drafting of 510(k) file in line with available FDA guidance document. |
I3C |
||
|
11 |
Review of Risk analysis, Equivalent device data, Biocompatibility Test/Safety test protocols |
CLIENT + I3C |
||
|
12 |
Review of Labels, User Manual/IFU, Shelf-life records/lifetime calculation, and pre-clinical study evidence |
CLIENT + I3C |
||
|
13 |
Pre FDA 510k Submission |
CLIENT + I3C |
||
|
PHASE III |
14 |
Compilation of 510(k) file by incorporating the pre-submission comments |
I3C |
90 Days |
|
15 |
Compilation of Pre-clinical and Biocompatibility and Safety testing |
I3C |
||
|
16 |
Compilation and release of the final draft |
I 3 C |
||
|
17 |
Review submission file |
I3C |
||
|
PHASE IV |
18 |
US Agent Appointment |
CLIENT |
20 Days |
|
19 |
Review payment |
CLIENT |
||
|
20 |
eSTAR submission of file to FDA |
I3C |
||
|
21 |
Receipt of acknowledgement |
CLIENT |
||
|
22 |
Wait for the review comments |
CLIENT |
90 Days | |
|
PHASE V |
23 |
Modify the submission file and provide additional supporting documentary evidence as per FDA review comments |
CLIENT + I3C |
60 Days |
|
24 |
Re-submission |
I3C |
10 Days | |
|
25 |
Wait for the review comments or FDA 510(k) clearance letter |
CLIENT |
90 Days | |
FDA 510K Submission Checklist
|
510K Section |
Topic |
510k file Contents |
| Section 1 | Medical Device User Fee Cover Sheet (Form FDA 3601) | |
| The Medical Device User Fee Cover Sheet is essentially a payment receipt containing basic information and the type of submission. | ||
| Section 2 | CDRH Premarket Review Submission Cover Sheet | |
| The CDRH Premarket Review Submission Cover Sheet is a document of around 5 pages, and it includes nine different sections. The initial sections A through D are quite simple and require basic information about the submission type, the reason for submission, and the applicant’s details.
However, Sections E and F can be tricky. It is recommended to take a moment to carefully read and understand these two sections. Section E asks for information about the predicate device, while Section F asks for information about your device.The problem is that both sections are listed one after the other, with no white space to separate them. Although there is a black line separating them, it can still be confusing. As a result, people often make the mistake of adding the predicate device name in Section F instead of their own product name. |
||
| Section 3 | CDRH Premarket Review Submission Cover Sheet | |
| The cover letter serves as an introduction to your submission, and it should be concise yet comprehensive. |
|
|
| Section 4 | Indications for Use Statement (FDA Form 3881) | |
| This section is crucial as it defines the intended use of your device. It’s important to align the level of specificity with that of the predicate device to avoid additional testing or revisions. Over-specifying can raise questions about safety and efficacy, potentially leading to extended time to market. | ||
| Section 5 | 510(k) Summary | |
| The summary containing the essence of your device and submission
Remember that the FDA makes this summary public within 30 days of their decision, so it’s important to balance the inclusion of sufficient details while meeting the FDA’s minimum requirements and maintaining strategic confidentiality. |
The summary should encapsulate the essence of your submission, including information from the cover letter, a comparison of substantial equivalence, and a synopsis of the testing performed |
|
| Section 6 | Truthful and Accuracy Statement | |
| This section is a declaration confirming the truthfulness and accuracy of the information in your submission. The FDA provides the exact wording for this statement. Your role is to include this statement verbatim in your submission, thereby certifying the integrity of your submission’s content. | ||
| Section 7 | Class III Summary and Certification | |
| If your device is Class II, this section will simply contain a statement: “This device is not a Class III device” |
If your device is Class III and does not require PMA, provide a statement and then include a summary addressing safety/effectiveness issues and supporting data. | |
| Section 8 | Financial Certification or Disclosure Statement | |
| If your device did not undergo clinical studies, include a single sentence: “No clinical studies were performed to test this device.” If clinical studies were conducted, you’ll need to complete the appropriate FDA form. There are two forms:
|
||
| Section 9 | Declarations of Conformity and Summary Reports | |
| This section involves declarations related to compliance with specific standards or regulations and summary reports of such compliance. Typically, this includes references to the standards or regulations your device conforms to and summary reports that demonstrate this conformity. | ||
| Section 10 | Executive Summary | |
| This section provides an overview of your device, including comparisons with the predicate device and a summary of all testing conducted. | ||
| Section 11 | Device Description | |
| This section in a FDA 510k submission requires a comprehensive description of your device, extending beyond the basic descriptions used in earlier sections.
The goal here is to give the FDA reviewer a thorough understanding of your device, laying the groundwork for the substantial equivalence Discussion. |
|
|
| Section 12 | Substantial Equivalence Discussion | |
|
Demonstrate step-by-step how your device is similar or equivalent to the predicate in terms of indications for use, technology, and performance.
|
Prepare a comparison table made in an easy-to-read table that highlights key similarities and differences without giving exhaustive information |
|
| Section 13 | Proposed Labelling | |
|
This section encompasses all labels and other written, printed, or graphic matter upon the immediate container of any medical device |
Include any relevant product information from your website, as the FDA considers this part of your labelling.
Specimen Label (Primary, secondary and or tertiary)
Name and Place of Business (21 CFR 801.1)
Exemptions may be granted in those instances where device labelling lacks sufficient space for required labelling under conditions. |
|
| Section 14 | Sterilization and Shelf Life | |
| The FDA 510k submission explicitly state if your device is non-sterile.
Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide (EtO) gas, vaporized hydrogen peroxide, and other sterilization methods (for example, chlorine dioxide gas, vaporized peracetic acid, and nitrogen dioxide).
Keep in mind that not all products require shelf-life testing. If your product is less likely to degrade over time, explain why shelf-life testing is not applicable. |
For sterile devices, provide evidence of sterility at the end of the product’s shelf life and that the device performs as expected throughout its shelf life. Include shelf-life testing results, which may involve accelerated ageing tests.
Device shelf-life claims must be supported with appropriate testing data. |
|
| Section 15 | Biocompatibility | |
|
If any part of your device comes into direct or indirect contact with patients, demonstrate that the materials used are safe and compatible with their intended use.
FDA may give an exception if your device is identical in material and manufacturing to the predicate device. |
Individual test protocols Reports of biocompatibility testing. |
|
| Section 16 | Software (Only applicable for Software device manufacturers) | |
| For SAMD or SIMD devices the 510k should give an appropriate definition of the software’s level of concern (minor, moderate, or major) and provide your rationale for this classification. | Documentation Level Evaluation A statement indicating the Documentation Level for the device and a description of the rationale for such documentation level.Software Description
Risk Management File
In compliance with ISO 14971 for Safety Risk Management.
Cybersecurity Risk Management File
Software Requirements Specification (SRS)
Traceability
System and Software Architecture Diagram
Software Design Specification (SDS)
Basic Documentation Level
Enhanced Documentation Level
Software Development, Configuration Management, and Maintenance Practices The summary information should include the following:
Enhanced Documentation Level Or
Software Testing as part of Verification and Validation
Basic Documentation Level
Enhanced Documentation Level
Software Version History
Unresolved Software Anomalies
Refer to the FDA’s guidance on software in medical devices and utilize IEC 62304, a recognized standard that outlines the medical device software lifecycle, incorporating a risk-based approach. |
|
| Section 17 | Electromagnetic Compatibility and Electrical Safety (Not applicable for non-active devices) | |
|
Assess and document how your device interacts with other electronic devices. This includes ensuring it does not cause or get affected by electromagnetic interference. Use IEC 60601-1-2 as a reference for EMC requirements and testing.
If your device has electrically powered components that are in patient contact, demonstrate their safety. Refer to IEC 60601-1 for general safety requirements, along with its amendments, to ensure compliance with safety standards. |
Statement of Intended Use Environment
Device design & Test level determination by consensus standards
Active implantable Medical Devices (AIMDs)
Special Environments
General guidance and rationale” of ANSI/AAMI ES 60601-1 and Clause 2.3.4 of AAMI CR500:2019 Basic Introduction to the IEC 60601 Series.
These criteria should be determined based on the medical device’s functions, modes, indications for use, intended use, and Essential Performance (if applicable).
Medical Device modifications to meet or pass EMC test, as a result of EMC test failure should be described with analysis.
|
|
| Section 18 | Performance Testing – Bench | |
|
This section typically encompasses the majority of your design verification and validation testing. It should include a comprehensive account of the bench testing conducted on your device. |
Test report summaries including the following element.
|
|
| Section 19 | Performance Testing – Animal | |
|
Not all devices require animal testing. If it’s relevant for your device, ensure you follow FDA guidance and potentially engage in a pre-submission process for feedback. Reference applicable guidance documents to understand specific animal testing requirements for your device category. |
Test Report
Test Protocol
Location of complete test report should be clearly marked in FDA 510k submission file. |
|
| Section 20 | Performance Testing – Clinical | |
|
The FDA will consider alternatives to clinical studies if supported by adequate scientific rationale. If clinical testing is necessary, determine whether your study is of significant or non-significant risk, complying with the relevant FDA regulations (21 CFR Parts 50, 56, and 812). |
Test Protocol
Test Report
|
|
We offer the best guidance and solutions for FDA 510k Submission to worldwide manufacturers and specification developers. Our services are time-bound and economical. We match prices. So partner with us and experience the difference firsthand.
Frequently Asked Questions
How should I prepare my 510k submission?
Preparation involves guidance document understanding, gathering all required documentation (Technical, manufacturing) conducting necessary testing, and ensuring that the 510k file meets all regulatory requirements. Engaging with a 510k regulatory consultant like I3CGLOBAL can help ensure a complete, compliant and fast submission.
Where and whom to submit 510k Q-submission?
You must submit an eCopy of your Q 510(k) submission under section 745(A)(b) of the FD&C Act. An electronic copy (eCopy) is an electronic version of your medical device submission stored on a compact disc (CD), digital video disc (DVD), or flash drive. Use the eCopy Validation Module to ensures your eCopy is formatted correctly. You must attach a paper copy of your company covers letter (including a signature) to the eCopy (CD, DVD, or flash drive).
A medical device submission package should be sent to the CDRH Document Control Center (DCC) at the following address:
U.S. Food and Drug Administration
Center for Devices and Radiological Health, Document Control Center (DCC) – WO66-G609, 10903 New Hampshire Avenue, Silver Spring, MD 20993-0002
What is Performance testing and Bench testing? How could help in faster 510k clearance
Performance data should be provided to help demonstrate the SE of your device to predicate the device. The data may include test results from engineering, bench, design verification, human factors, animal testing, and clinical studies and clinical trials. Tests should be conducted on all sizes and models of the device.
FDA classifies non-clinical performance testing under bench testing. These tests are performed by either a device manufacturer or a third-party testing facility (e.g., test laboratory), which includes all bench testing and will depend on the specifics of the actual device or device type. Non-clinical bench performance testing includes tests to evaluate mechanical and biological engineering performance (e.g., fatigue, wear, tensile strength, compression, etc).
The reviewer will require test report summaries, test protocols, and test reports. The test report summary ideally includes a brief description and summary of the various tests performed as part of bench testing. The complete test reports also should be included as part of the premarket 510(k) submission. Test protocols contain the testing methods, test objective, acceptance criteria, and data analysis plan. The contents of the test protocol can also be included as part of the test report and submitted as a single document.
How long the Q 510k submission process?
75-90 days (*21 days for urgent public health issues)
Day 1 Review Team
Day 5 Acknowledgement
Day 15 Acceptance Review
Day 30 – 40 Meetings Scheduled if needed
Day 70 FDA Feedback
Day 75 Meeting
Day 90 Meeting Minutes from Submitter
Day 120 FDA revisions to the meeting, if needed
If FDA determines that the information provided is insufficient, the request will be refused to accept, or RTA and the sponsor should provide the additional information, which will be logged in as an amendment to the Q-Submission
Can a applicant withdraw a 510k submission?
Yes, an applicant can withdraw a 510k at any time before the FDA makes a final determination. The review fee paid should collect back in 60 days.
How much is the total cost for a 510k submission?
- FDA Review Fee
- Documentation and US Agent Fee
- Biocompatibility / Electrical Safety (if applicable)
- Performance tests
- Validations
- Shelf life / lifetime etc.
What happens if my 510(k) submission is not accepted?
How long does the 510k submission and review process will take?
The FDA generally targets to review and make a determination on a submission within 90 calendar days. However, the timeline may fluctuate based on the complexity of the device and the quality of the submission.
Any specific restrictions on the file size for a 510k submission to the FDA?
There are no specific restrictions on the file size for a submission to the FDA, but there are practical considerations to keep in mind.
All submission must be made electronically through the FDA’s Electronic Submission Gateway (ESG). While there isn’t a strict file size limit, large files can take longer to upload, and there may be technical challenges related to very large submissions.
Any specific file structure for a 510k submission?
The submission should be well-organized, typically divided into multiple files or sections. This not only makes it easier to upload but also helps the reviewers navigate the document efficiently. Common practice is to break down the submission into different sections.
Any practical tips regarding 510k submission file upload
Compressing files where possible (e.g., optimizing PDFs) can help manage overall submission size. Always check the final 510k submission files to ensure they meet the FDA’s requirements, including ensuring that all links work, files are readable, and the submission is complete.