Quick Contact

Medical Device Single Audit Program (MDSAP)
The Medical Device Single Audit Program (MDSAP) was developed by a group of medical device regulators, the International Medical Device Regulators Forum (IMDRF), to allow third-party auditors to conduct a single audit of a medical device manufacturer.
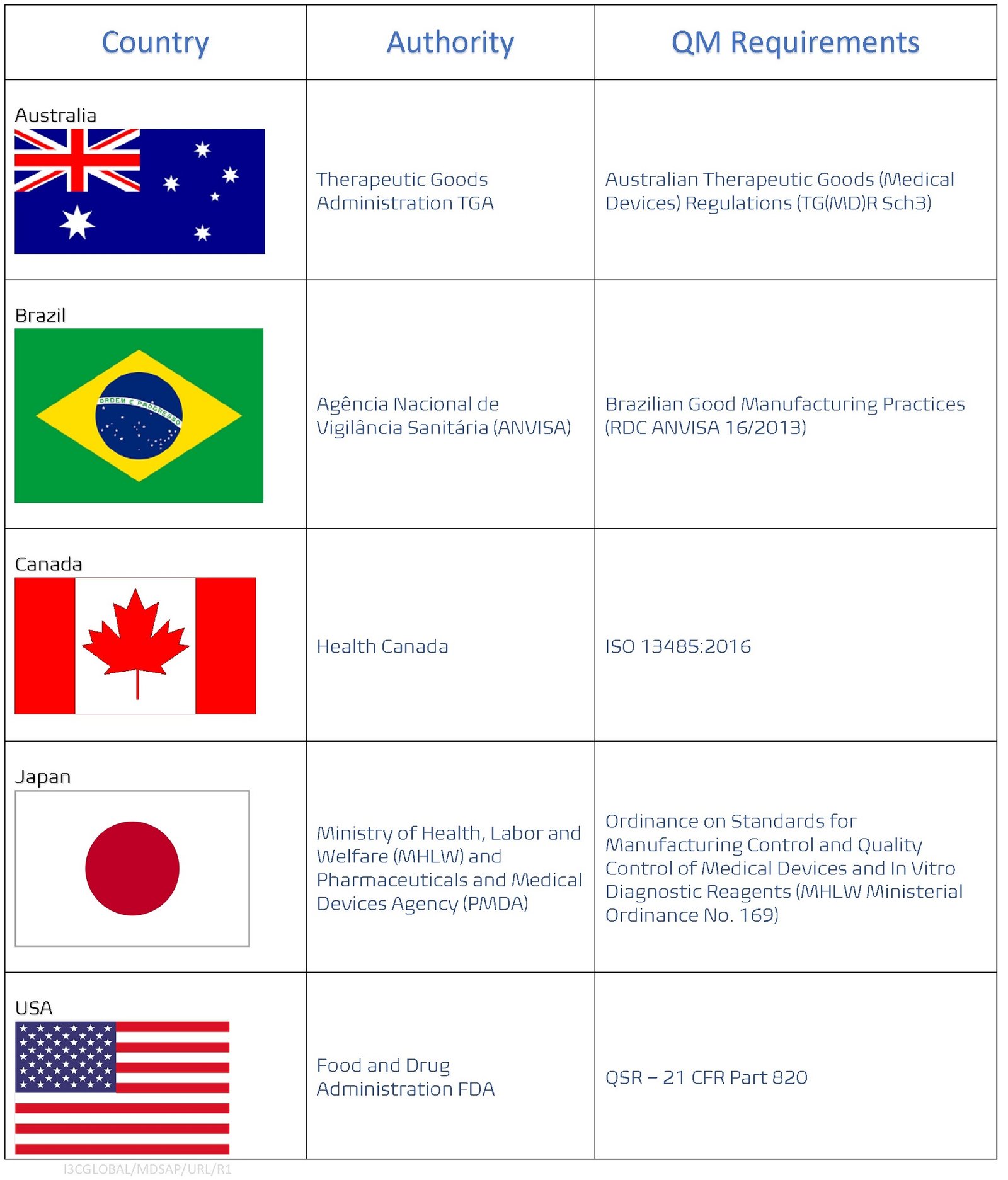
The MDSAP participants are Australian Therapeutic Goods Administration (TGA), Brazil’s Agência Nacional de Vigilância Sanitária (ANVISA), Health Canada, Ministry of Health and Labour and Welfare (MHLW) of Japan, and the US Food and Drug Administration (FDA)
MDSAP Structure is framed to use ISO 13485:2016 in line with Good Manufacturing Practice (GMP) requirements of the various regulatory authorities.
MDSAP is aligned with ISO13485 and includes seven major processes:
- Management,
- Measurement, Analysis, and Improvement,
- Design and Development,
- Production and Service Controls
- Purchasing,
- Device Marketing Authorization and Facility Registration, and
- Medical Device Events and Advisory Notices Reporting
Benefits of MDSAP Certification
- Avoid multiple audits covering multiple standard requirements by multiple Certification Bodies in a year.
- Efficient, single audit scheme minimizes business disruptions, reduces costs and saves time.
- Much faster market penetration where traditional regulatory oversight can cause significant delays.
- Unified integrated documents can be maintained on-site with the manufacturer thereby reduce lot of paper work and duplication.
Role of MDSAP Consultants
- MDSAP qualified lead auditors and experienced consultants.
- On-site and Off-site documentation support.
- MDSAP Awareness and IQA Training to employees.
- Initial MDSAP Internal Audit support.
MDSAP Consultation Fees
- GAP Assessment – 700 USD/Man-day.
- Guidance / Support for Fixing GAPS – 400 USD/Man-day.
- Internal Audit – 600 USD/Man-day.
For a detailed quote Click Here >>