FDA 510k Consultants For Medical Device
Our FDA 510k Consultants help you navigate the complete process for Class I, Class II, and Class III medical device by thoroughly understanding the devices and by identifying any possible pitfalls. Qualified and experienced technical experts assure faster clearance.
If you’re looking for best FDA 510k Consultants and experts, your search is over. We can help you with all of your 510k needs. We specialize in the following areas:
- 510k Documentation
- US Agent Service
- US FDA 510k review and coordination
- FDA Registration
- FDA UDI (Unique Device Identification) compliance assistance
- FDA label requirements assistance
- FDA quality systems assistance
Failing to follow the FDA’s regulations could result in costly consequences, so it’s important to understand how to effectively communicate with them. I3CGLOBAL FDA 510k Consultants can help you navigate the FDA’s regulatory requirements to market your device. The 510k submission process is the means by which medical device manufacturers can demonstrate that their device is of an equivalent device with regard to safety and effectiveness.
An FDA 510k is a premarket submission made to the FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device (21 CFR 807.92(a)(3)) that is not subject to premarket approval. The criteria for substantial equivalence are specified in 21 CFR 807.87 and 807.81.
We have an entire section at I3CGLOBAL devoted to assisting you with the FDA 510k clearance procedure. Our FDA 510k consultants will assist you in determining whether your product requires 510k clearance, and if it does, they will walk you through the process every step of the way. You’ll never feel alone in your efforts to bring your product to market. The best part? We offer all this at rates that won’t break your budget.
Our Mission is to help companies navigate the FDA’s complex 510k approval process, which can be quite difficult to navigate for small and mid-sized medical device firms. We are committed to delivering the most effective and innovative medical device solutions for surgical procedures under FDA 21 CFR 801 and the 510k process. We work with each client to define the most efficient approval pathway.
If you’re a manufacturer or specification developer seeking approval for a new medical device, you’ll need to submit a 510k application to the FDA.
We specialize in assisting medical device manufacturers and specification developers in efficiently preparing and submitting their 510k files to the FDA, reducing the likelihood of receiving an initial “Refuse to Accept” (RTA)
FDA 510k Consultants Roles and Responsibilities
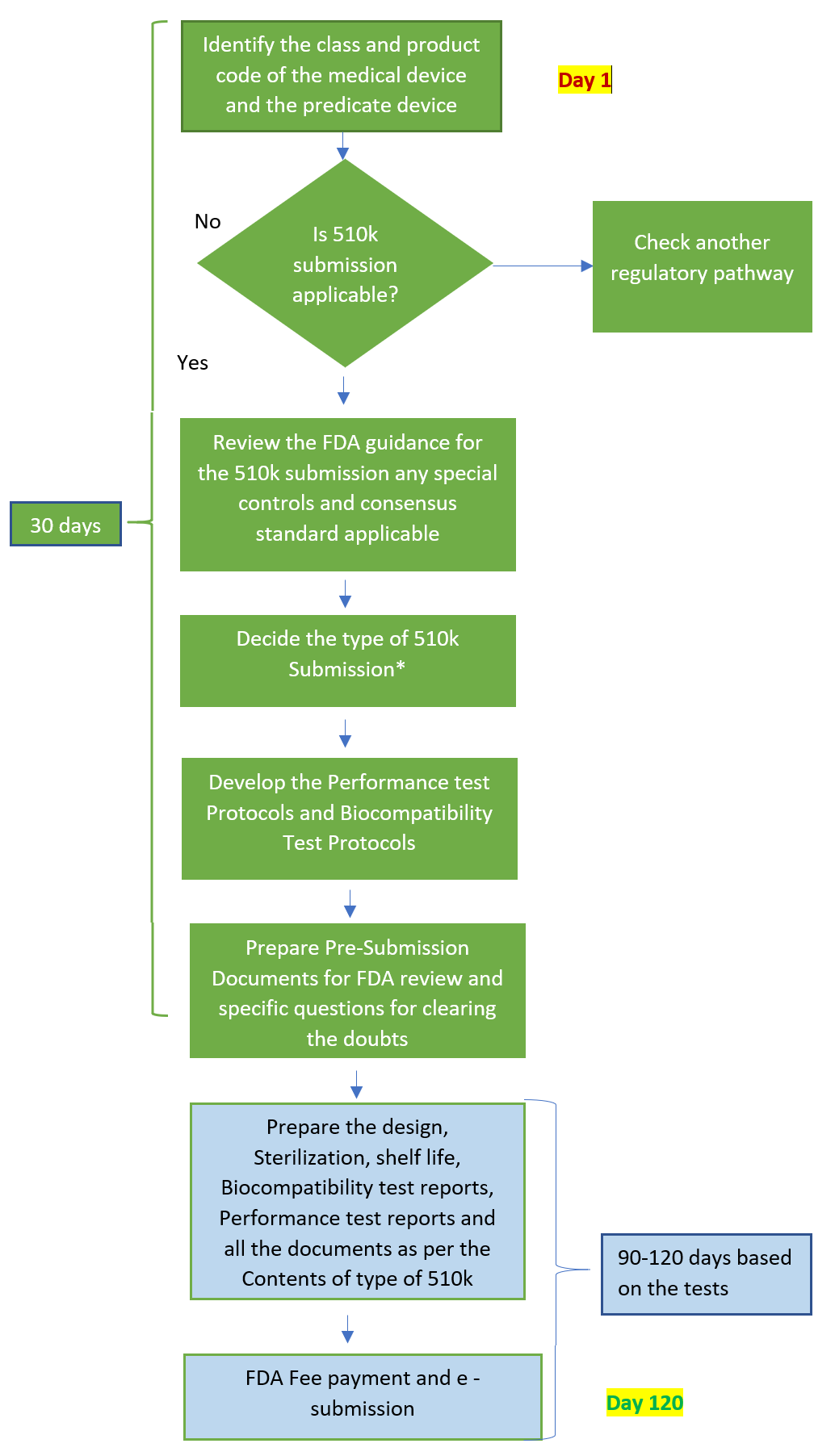
- Identify device class, product code, and regulation number.
- Appropriate predicate device Identification in FDA 510k consultation with manufacturer
- Identify appropriate guidance and control documents.
- Choosing an appropriate type* of US FDA 510k submission
- Prepare Indication for use statement.
- Declaration of Conformity
- Identification of biocompatibility tests
- Identification of performance test
- Sampling Plan
- Requirements for stability studies
- Review of the risk management file
- Sterilization requirements and appropriate documentation
- Labeling requirements and review of label
- US FDA 510k summary and predicate comparison
- Identify any clinical data/ test requirements.
- Device-specific documentation requirements- includes Software, EMC, and Electrical safety.
- Review of final 510k file
- Pre-submission (Q-submission)
- Refuse to Accept policy for 510k acceptance review.
- E-copy submission
- Interact with FDA during initial review, substantial review and interactive review.
- Device Listing and Registration
- US Agent Services
Do you need an email containing full details within 2 minutes?
Advantages of FDA 510k Consulting Services
- Confirm the medical device requires US FDA 510k and is regulated under the FDA.
- Identification of the perfect 510k pathway to get clearance.
- Choosing a suitable predicate device to establish substantial equivalence.
- Previous experience and knowledge of FDA requirements make the total process faster.
- Well-organized Documentation.
- Review of the FDA 510k documentation and identify compliance gaps.
- Knowledge of the latest trends and the current regulations in requirements for the submission.
- Helps in identifying appropriate performance tests, consensus standards, and the requirements of other Clinical/non-clinical studies.
- Guidance on every stage of 510k file development
- Timely preparation of file and submission to FDA.
- Support during the Initial, substantial and interactive review period.
- With the customer till clearance is done.
FDA 510k Approval Timeline
While reading the FDA website, it is clear that the FDA 510k Clearance time set by the FDA authorities is within 90 working days. The possible delay due to RTA and AI, their internal review clock stops and does not begin again until you rectify and inform the reviewer. Our experience with the majority of medical devices’ 510k clearance was obtained in 08-10 months.
Types of FDA 510k Consulting Services
TYPE 1 (Total Package)
- Reconfirm the classification, product code, and regulation number.
- Select the proper predicate for your 510k submission.
- Preparation of 510k (We FDA 510k consultants prepare and maintain the file until clearance)
- Act as US Agent
- Electronic & hard copy submissions
- Answering to review comments and File re-submission.
TYPE 2 (510K Review Service)
- Verify Application and contents.
- Review of client prepared 510k file and submit GAP assessment report.
TYPE 3 (US Agent + Submission & FDA Communication)
- Act as the US Agent
- The communication channel between the FDA and foreign facility
- E-copy and hard copy submissions to the FDA
Working with FDA 510k consultants like I3CGLOBAL helps manufacturers prepare and respond to FDA queries more efficiently and effectively, ultimately speeding up the 510k approval process and bringing their medical devices to market faster.
Frequently Asked Questions
What is the timeframe for preparing a 510k file for an implant device?
The time needed to prepare a 510k submission for an implant device can vary depending on the device code and subsequent codes. Generally it takes 90 to 150 days.
What is the timeframe for preparing a 510k file for a SAMD?
The time needed to prepare a 510k submission for a SAMD can vary depending on how well the developer implemented IEC 62304. It also depends on the device code and subsequent codes. Generally, it takes 90 to 150 days.
What is the timeframe for preparing a 510k file for a IVD?
The time needed to prepare a 510k submission for an IVD can vary depending on how fast the manufacturer can produce performance evaluation reports and supporting evidence. It also depends on the device code and subsequent codes. Generally, it takes 120 to 180 days.
What is the timeframe for answering FDA quries?
Generally, it takes 30 days each time the FDA sends the queries.