UKCA Marking Medical Devices!
The UKCA marking is a new product marking that is used for medical devices and in vitro diagnostic devices primarily along with certain other goods being placed on the market in Great Britain (England, Wales, and Scotland).
Contact Us
UKCA Marking Medical Devices Overview
UKCA Marking medical devices is a valid indicator that a medical device conforms to relevant UK Regulations. The United Kingdom conformity assessment marking is mandatory for medical devices sold in Great Britain (England, Wales, and Scotland) post-Brexit. The UKCA Mark is the UK equivalent of the EU CE Mark.
The United Kingdom’s Medicines and Health Care Products Regulatory Agency (MHRA) published new guidelines to regulate medical devices after Brexit. The UKCA will not be recognized in the EU, EEA, or Northern Ireland, and products still require CE Certification for sale in these markets. The manufacturer or their authorized representative will be responsible for affixing the UKCA certification to the product, following the same principle as for CE marking but for the UK market.
A third-party evaluation procedure by a UK-approved body is required for UKCA Marking sold in the UK. This procedure is comparable to that used in the European Union for CE Certification, in which the EU-authorized Notified Body assesses compliance. The MHRA has stated that the UKCA Mark will be based on the IVD, MDD, and AIMD Directives until June 30, 2023, rather than the EU MDR 745/2017 and EU IVDR 746/2017.
UKCA Mark Consultant
UKCA mark consultant can provide technical expertise and assistance with compliance and MHRA registration to manufacturers who need to apply for UKCA Certification or compliance with the UK Conformity Assessment (UKCA). Here are some reasons why manufacturers might consider working with UKCA consultant.
- Confirmation device models and scope of UKCA Marking
- Identification of Medical and in vitro device risk classes
- Identification and finalization of test standards and device-specific standards
- Identification of the assessment route for CE Certification
- Systematically organize and arrange Technical Documentation and files.
- Coordination with CB during the review
- Clinical Evaluation Report documentation covering PMS, PMCF, and PSUR
- Risk Analysis
- Medical Device Quality Management System Implementation
- UDI support
- GMDN confirmation
UKCA Technical File
Members of the QA and regulatory teams frequently struggle to discover the requisite number of medical device technical files for devices slated for UKCA Marking. The correct segmentation of technical files based on intended usage, application, and GMDN Code is critical for the file’s quick processing. It is advised to remember that the medical device technical file or documentation is all about your device to establish safety and performance. It means you cannot combine products with different intended uses, different classes, different construction materials, or even different designs.
- Device Intended use and Indication of use
- Site of application
- Design change or major constructional changes
- State of the art
- Addition of multiple directives or regulations
- Evaluation of accessories, components, and software/firmware
Conformity Assessment Bodies
The UK Notified Body accreditations will be revoked on December 31, 2020, and their CE marking will no longer be acceptable for placing medical devices and in vitro diagnostic equipment on the UK market. After December 31, 2020, UK-based Notified Bodies will automatically become UK Conformity Assessment Bodies, and their CE Certificates will no longer be valid.
The common question raised by EU manufacturers is whether the CE Mark Certificate issued by an EU Notified Body will be accepted by a UKCA UKCA-approved body and utilized to produce a UKCA Certification for the same medical device. The response is that if EU Notified Bodies are willing to share information with UKABs when the certificate holder asks for it, UKABs will be able to provide UKCA Certification without having to repeat the entire certification process. Cooperation between EUNB and UKAB is vital; thus, we assess the possibilities on an individual basis and guide manufacturers accordingly.
It’s important to remember that conformity assessment entails an audit of the manufacturer’s quality management system as well as a review of technical documentation related to the products to be CE Marked for specific criteria. As a result, if your EU-notified body isn’t cooperating with a UKAB, additional on-site audits and technical documentation reviews will be required, and manufacturers will have to pay a hefty fee for the process.
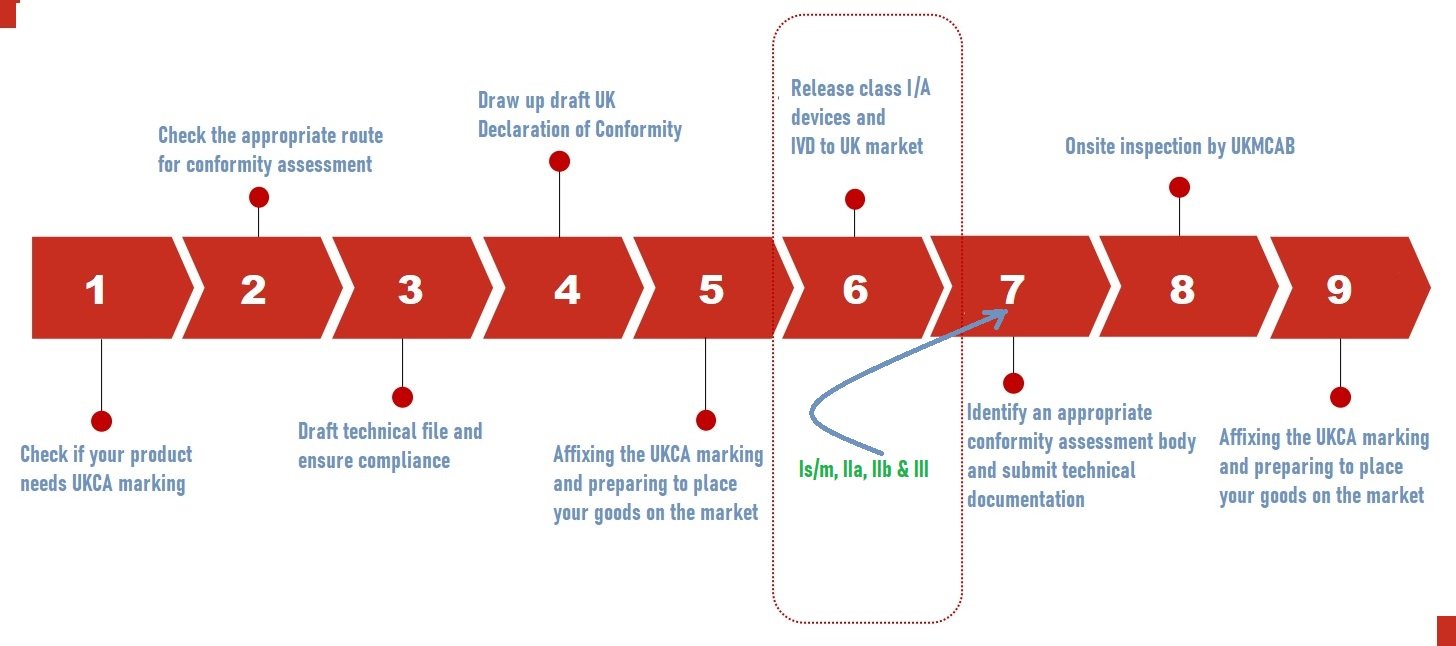
UKCA Conformity Assessment Steps
- Identify the directives and standards that apply to you.
- Double-check the prerequisites.
- If relevant, conduct pre-clinical, safety, and performance investigations.
- Prepare a technical file and a compliance statement.
- The technical material should be submitted to the Conformity Assessment Body (UK CAB).
- The onsite inspection was followed by a review of the technical files.