Quick Contact

FDA 510K Third Party Review
The FDA came up with the 510k third party review to make it easier to deal with the large number of 510k requests from medical device manufacturers seeking to determine whether their manufactured medical devices are substantially equivalent or not substantially equivalent. FDA’s third-party review program is targeted at decongesting FDA’s administrative workload and intends to increase the speed of 510k decisions.
Accredited FDA Third Party Assess Organizations are permitted to review some low-to-moderate risk medical devices under the 510k Third Party Review Program, which offers medical device manufacturers a voluntary alternative review method. With the program, the FDA will be able to concentrate its resources on higher-risk devices and make 510k decisions more quickly while still keeping control over the review of lower-risk devices that qualify for third-party review. This program is formally known as the Accredited Persons Program.
Use of this program is voluntary. The FDA accepts about 50 percent of 510(k)s that qualify for this program. There is no separate payment (i.e. user fee) to the FDA; the only payment made under the program is between the 510(k) submitter and the FDA third party Review organization.
The four basic steps of FDA 510k Third Party Review
It’s important to note that the 510k process is not a one-size-fits-all process. The specific requirements and steps may vary depending on the device code, regulation number, and intended use. It’s important to work closely with the I3CGLOBAL consultants throughout the process to ensure all requirements are met.
Determine eligiblity
To determine if a FDA 510k submission is eligible to be reviewed under the Third-Party review Program, the 510k submitter can do any of the following:
-
- Review the list of eligible devices for the device product code (most Class I and Class II devices are eligible);
- Search the FDA’s Device Classification Database.
- Contact a FDA Third-Party review organization or fill 510k Request Proposal Form
- Contact the FDA at 3P510k@fda.hhs.gov.
Find 3rd Party 510k review organization
A 510 (k) submitter can use either of the methods below to find and contact organizations that can review their 510(k) submissions:
-
- Access the List of Devices for FDA Third Party Review page and then:
- Click on a device type at the bottom of the page. This will display a table with regulation names and product codes; and
- Click on the product code of the device. This will display additional information for the product code, including a list of any third party 510k review organisations that are eligible to review that type of device.
- Review the list of 510k review organisations (also referred to as Accredited Persons). The list of FDA third party review organisations shows the devices each organisation is accredited to review, and their contact information.
- Access the List of Devices for FDA Third Party Review page and then:
Receive Quote
The fee for a third party review is determined by the agreement between the 510k submitter and the Review Organisation. The 510k submitter pays the fee directly to the third party 510k Review Organization. The FDA does not collect a user fee for third-party submissions.
Submit the 510(k)
- Name of the third party 510k review organisation.
- Name and contact information of the person assigned to the review; and
- Device trade name.
The complete 510k submission, including the supporting data, summaries and analysis in the format requested by the 3P510k review organization.
The same standards used by the FDA to evaluate 510k submissions are used by third party 510k Review Organizations. To make sure a third party 510k review organization is following the most recent standards and recommendations applicable to that type of device, the review process may involve early involvement with the FDA.
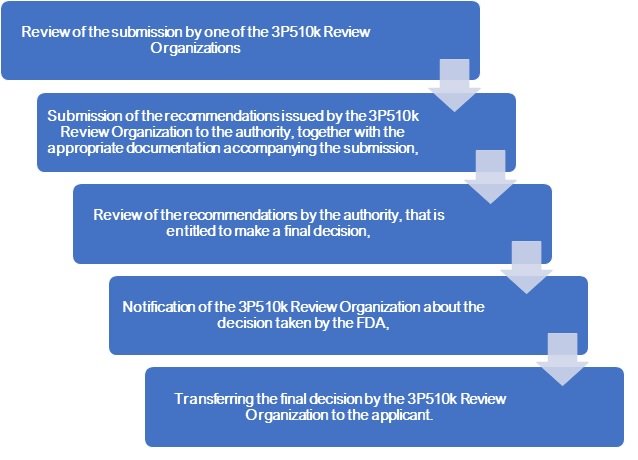
It may also include requests for additional information from the 510k submitter. After the FDA third party review Organisation is satisfied with its review and has documented all the necessary information for the submission, it sends the submission to the FDA including the original 510(k) submission, the third party 510k Review Organisation’s review, and a recommendation of either substantially equivalent (SE) or not substantially equivalent (NSE).
The FDA makes the final determination on the 510k-submission based on the FDA third party review and recommendation received from the third party 510k review organisation. The FDA’s review timeframe for a MDUFA decision is within 30 days after receiving the recommendation of a 510k review organisation.