
ISO 13485 Consultants
Medical device organizations must appoint experienced ISO 13485 consultants with knowledge of all risk-class devices. The regulatory requirements in the medical device industry are being constantly reviewed and amended accordingly, with an increasing emphasis on patient safety.
The ISO 13485 Consulting organization must manage and extract value from the vast amounts of structured and unstructured information and data, ensuring their organization stays updated and compliant with regulatory, regional, and local requirements.
The organization should implement effective systems to track and monitor safety and benefit-risk information throughout the life-cycle of its products in line with ISO 13485 requirements.
I3CGLOBAL ISO 13485 consultants identify and integrate product-specific regulatory requirements such as MDR, IVDR, or FDA 510k during the implementation. This will help later when the organization plans for CE marking or 510k clearance.
Do you need an email containing full details within 2 minutes?
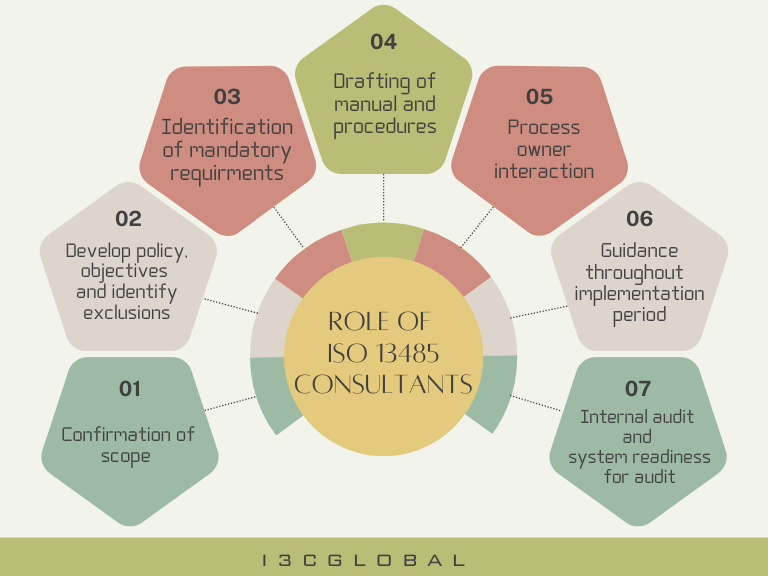
ISO 13485 Consultants Roles and Responsibilities
ISO 13485 Consultants help manufacturers efficiently plan and implement medical device quality management systems in accordance with local government regulations and related product certification requirements by:
- Identifying the QMS standard requirements
- Identifying all legal and regulatory requirements based on the product’s classification.
- Planning for establishing the standard requirements stage-wise.
- Developing and defining the processes and their documentation
- Providing training to the QMS team
- Implementing the QMS
- Conducting internal quality audits
- Organizing Management Review Meetings
- Developing a continual improvement approach for the whole QMS
- Evaluating the effectiveness of the QMS implemented in the organization
We are ISO 13485 consultants and regulatory experts for MDR, IVDR and FDA. We guide and understand the organization’s requirements in line with the product compliance team for proper implementation
ISO 13485 Quality Manual
A quality manual is considered the mother document in a QMS system designed for medical device manufacturers. If you are manufacturing medical devices for use in the EU region with CE Marking, you will need to prove compliance with EN ISO 13485:2016. ISO 13485 certification provides flexibility in how organizations choose the structure of the quality manual or how it should be circulated.
ISO 13485 Mandatory Procedures
ISO 13485-implemented organizations can have many types of procedures, ISO standard operating procedures, work instructions, or standard operating procedures, work instructions, or protocols based on the processes and activities.
ISO 13485:2016 Throughout the standard, in multiple places, it has used words such as “shall, should, must, documented procedures,” which directly warns the organization to keep documentation in the form of procedures. These procedures are commonly called mandatory procedures.
PDCA cycle (Plan – Do – Check – Act)
ISO 13485:2016 follows the PDCA cycle (Plan, Do, Check, and Act), which helps to adopt a drive for continuous perfection in the processes of the organization. This approach can improve any process by dividing it into smaller steps, is effective for the implementation of QMS, and helps to improve the whole product realization process.
Also, it provides a range of solutions to problems and helps to select the best one for implementation. This method helps with the proper allocation of resources, avoiding waste, for the successful implementation of the solutions.
Steps of ISO Implementation and Certification
ISO 13485:2016 certification, an organization must be able to provide medical devices and related services that consistently fulfil customer and regulatory criteria. This is done by demonstrating its capacity to implement a quality management system.
The design and development, manufacture, storage, distribution, installation, or servicing, as well as the design and development of related services like technical assistance, are all activities that these firms may be involved in at one or more phases of the life cycle.
- Planning the quality system
- Meeting regulatory requirements
- Implementing design controls
- Documents, records, and training
- Management processes
- ISO 13485 Consultants Audit
- Third-party or CB audits
We are ISO 13485 consultants and regulatory experts for MDR, IVDR and FDA. We guide and understand the organization’s requirements in line with the product compliance team for proper implementation
Frequently Asked Questions
Do you provide online support for ISO 13485 Implementation?
Yes, we provide online and onsite service based on customer requests.
How long to implement a strong ISO 13485 system?
With the help of process owners, the implementation and certification can be completed in 3-4 months.
Which all cities you provide onsite service?
Service is available in Boston, Bangalore, Chicago, Chennai, Cambridge, Dusseldorf, Delhi, Dallas, London, Mumbai, Newyork, Los Angeles, Minnesota, Houston, San Diego, Seattle, Nashville, and Salt Lake City
