Quick Contact

Class A IVDR Classification
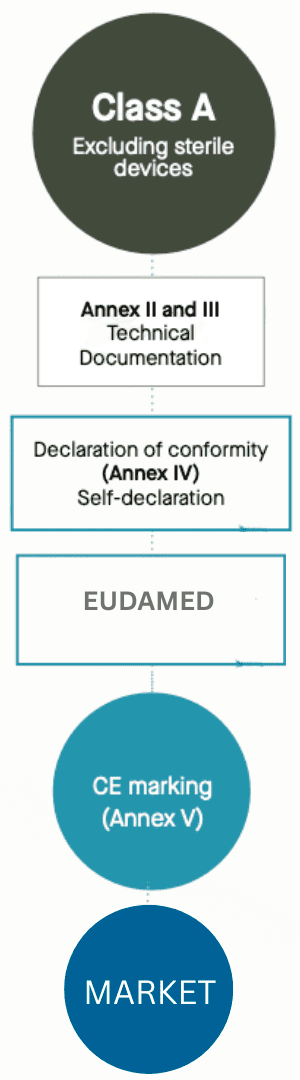
Class A IVD devices are considered low-risk in vitro diagnostic medical devices under the EU IVDR (2017/746)classification system. These are typically instruments, accessories, lab equipment, and other IVD products that pose minimal risk to patients and the public when used correctly. While Class A devices have simpler regulatory pathways compared to higher classes, manufacturers must still ensure compliance with IVDR requirements, including quality management systems, technical documentation, performance evaluation, and proper labeling
I3CGLOBAL provides end-to-end Class A IVDR consulting – fast compliance, clean documentation, and smooth certification execution. Contact us today
Key Requirements for Class A IVDR
-
Confirm Risk Classification as low-risk IVDs with minimal impact on individual and public health. Commonly includes general laboratory accessories and low-risk instruments.
-
Complete Technical Documentation (Annex II) with the help of a regulatory consultant and must be prepared and kept up to date.
-
Safety & Performance requirements of the devices must demonstrate compliance with General Safety and Performance Requirements (GSPRs) – Annex I and must be updated in the technical documentation
-
Conformity Assessment (Route A) and manufacturers may self-declare conformity without Notified Body involvement
-
Implement a Quality Management System (QMS) and maintained, typically aligned with EN ISO 13485 requirements
-
If the device has electrical, wireless, or mechanical components, compliance with EMC, RED, or Machinery Regulations may also be necessary and evidences must be attached to the technical documentation.
How to Upgrade Previously IVDD Class A / General Devices to IVDR Class A
Devices under the old IVDD 98/79/EC framework, including many low-risk laboratory products and general IVD accessories, were classified as Class A (General). With the implementation of the IVDR (EU 2017/746), the regulatory requirements have increased significantly even for Class A devices. Manufacturers must now comply with the requirements mentioned above and follow continuous post-market surveillance and vigilance activities.
Do you need an email containing full details about IVDR Classification within 2 minutes? Share your email below: Privacy Policy>>
Frequently Asked Questions
Can I3CGLOBAL help with Class A IVDR Certification?
Yes, I3CGLOBAL provides complete consulting support including technical file preparation, labelling review, EU Representative and EUDAMED, ensuring smooth and economical approval.