European Medical Device Nomenclature
Manufacturers will use the European Medical Device Nomenclature (EMDN Code) when registering their medical devices in the EUDAMED database.
Article 26 of EU 2017/745 Regulation on Medical Devices (MDR) and Article 23 of EU 2017/746 Regulation on In-vitro Diagnostic Devices (IVDR), the European Medical Device Nomenclature (EMDN) aims to support the functioning of the European database (EUDAMED). Among its various uses, it will be utilised by manufacturers for the registration of medical devices in EUDAMED, where it will be associated with each Unique Device Identifier – Device Identifier (UDI-DI).
It would facilitate effective market surveillance operations and facilitate device traceability throughout the supply chain. The European medical device nomenclature will be free of cost to the manufacturers and other natural/legal persons required by the regulations to use this nomenclature.
How is the EMDN structured?
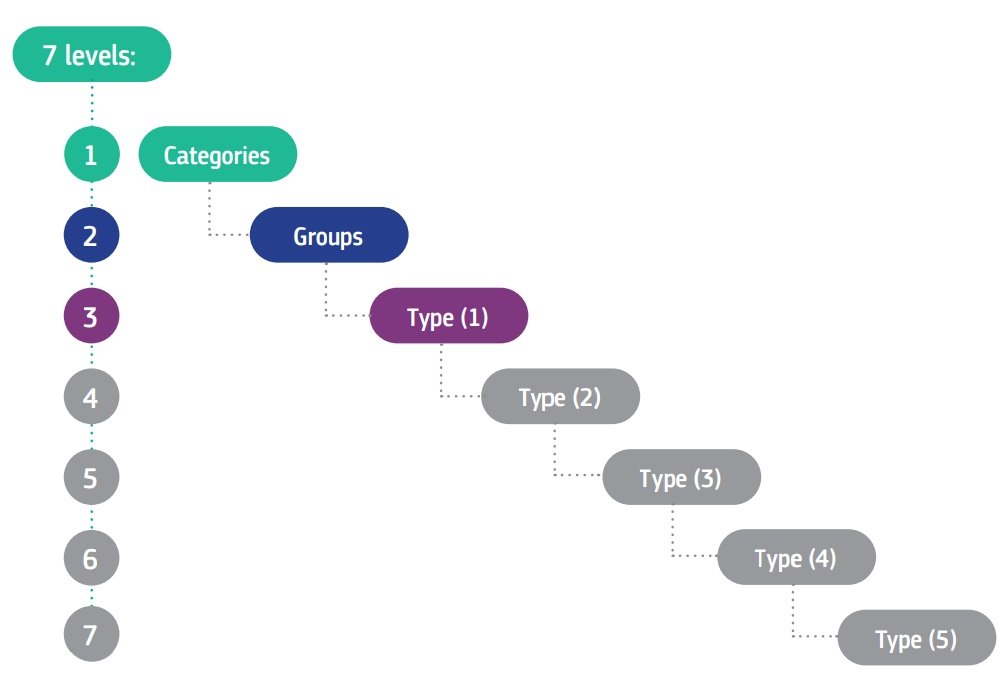
The EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree. It clusters medical devices into three main levels:
- Categories: the first hierarchical level,
- Groups: the second hierarchical level,
- Types: the third hierarchical level (which expands into several levels of detail (1°, 2°, 3°, 4° and 5°), where necessary.
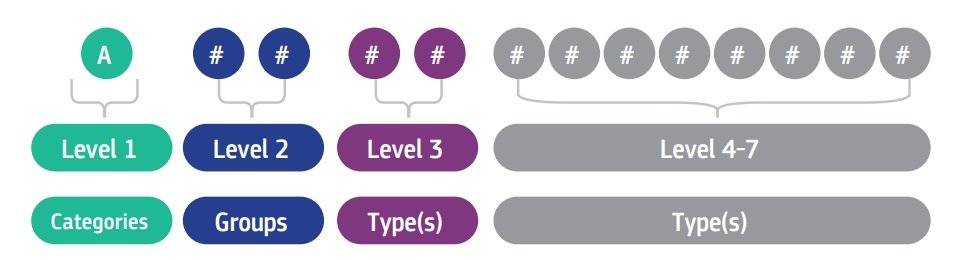
Each alphanumeric code begins with a letter referring to the ‘CATEGORY’ for which the device falls, followed by two numbers indicating the ‘GROUP’ and a series of numbers which refer to the ‘TYPE’. The maximum number of digits is set at 13.
During EUDAMED registration or technical documentation, manufacturers always assign the most granular and terminal term available (lowest level in the tree) to their device.
EMDN codes are now mandatory for every medical device within the EU, to be included either during the preparation of technical documentation, the NB application process, or the registration on EUDAMED

Currently we are using GMDN code in Technical file, as said in above content that The European Medical Device Nomenclature (EMDN) will be the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database, so once EMDN is established manufacturer would have to include EMDN along with Unique Identification Number in Technical File
The medical devices nomenclature is a coding system and used for classification and identification of all medical devices generically. There are several nomenclature systems presently existing and they are used by different groups of professionals, based on their specific needs. As the European Commission wants to maintain EUDAMED, as per the new regulations, it requires a unique nomenclature system, that can be freely available to all the groups.
For the same, they want to develop European Medical Device Nomenclature system, by referring the CND and GMDN. At present the process is going on.
Mentioning the EMDN in technical documentation and labels, will be beneficial for the better communication between individuals and organizations.
For registering medical devices under MDR, the manufacturer shall use European Medical Device Nomenclature (EMDN) with the help of the upcoming EUDAMED database.
Currently, the CND is being revised to enable the release of the first version of the EMDN. The commission will map the connection between the two nomenclatures GMDN and EMDN will be incorporated in the EUDAMED database as a “searching tool” to ease the research to the operators.
I don’t know what to comment on because I don’t understand what that means yet