Periodic Safety Update Report (PSUR) Services
The Periodic Safety Update Report is a new requirement outlined in MDR 2017/745 Article 86 and IVDR 2017/746 Article 81. We assist manufacturers of all sizes- small, medium, and large by offering expert guidance on PSUR documentation. Our extensive experience and dedicated team are well versed in the NB expectations regarding PSURs.

Quick Contact
What is a Periodic Safety Update Report (PSUR)?
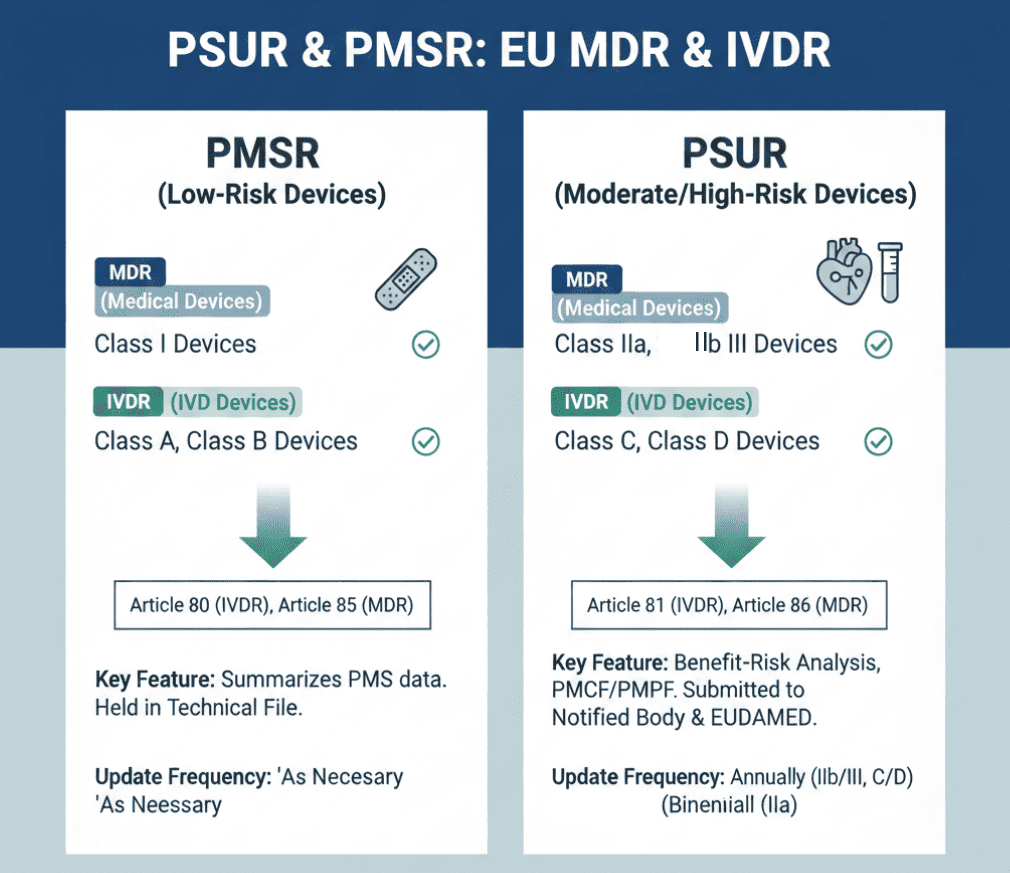
A Periodic Safety Update Report (PSUR) is a documented summary of data collected from post-market surveillance activities. Unlike the Post-Market Surveillance Report (PMSR) used for low-risk devices, the PSUR is mandatory for moderate to high-risk medical devices and IVDs. The primary objective of the Periodic Safety Update Report is to provide a transparent overview of the device’s safety profile, including:
⊕ Summary of results from Vigilance and PMS data.
⊕ Conclusions of the benefit-risk determination.
⊕ Main findings of the PMCF (MD) or PMPF (IVD).
⊕ Sales volume data and an estimate of the user population characteristics.
Expert MDR and IVDR PSUR Writing Services
Struggling with complex PSUR timelines and Notified Body expectations?
The Periodic Safety Update Report (PSUR) is no longer a “check-the-box” exercise. Under MDR 2017/745 and IVDR 2017/746, it is a high-stakes technical document that can determine the market life of your device. PSUR is the most critical for your Technical Documentation. A poorly drafted Periodic Safety Update Report is one of the leading causes of non-conformity during Notified Body audits. It isn’t just a summary; it’s an argument for your device’s continued safety.
The I3CGLOBAL Advantage: Why Manufacturers Trust Us
- Notified Body Alignment: We write reports that anticipate the specific questions from leading Notified Bodies.
- Cross Functional Precision: We ensure your PSUR perfectly sync with your Clinical Evaluation Report (CER), Risk File, and PMCF.
- Data-to-Insight Conversion: We don’t just list your sales and complaint data; we perform the trend analysis and benefit-risk evaluation required by MDR Article 86 and IVDR Article 81.
- Regulatory Peace of Mind: Never miss a submission deadline. We manage the annual and biennial update cycles for your entire product portfolio.
At I3CGlobal we bridge the gap between raw post-market data and a Notified Body-ready PSUR. We help manufacturers secure their CE certification by delivering data-driven, audit-proof reports.
Attention!!
A common pitfall in Periodic Safety Update Reports is a lack of integration between the Post Market Clinical Follow-up (PMCF) findings and the final Benefit-Risk conclusion. Under EU MDR, your PSUR must not only list safety data but also critically evaluate if the device’s benefit-risk profile has changed. I3CGLOBAL’s medical writers ensure that your technical documentation provides a cohesive narrative, directly addressing Notified Body expectations regarding the ‘Acceptability of the Benefit-Risk Ratio’ – a critical factor for maintaining your CE Certification.
Comprehensive PSUR Solutions for MDR & IVDR
MDR Periodic Safety Update Report for Class IIa, IIb, III manufacturers, the report requires a rigorous assessment of the benefit-risk ratio. Our experts specialize in:
⊕ Synthesizing Vigilance and CAPA data.
⊕ Evaluating PMCF findings to support continued clinical safety.
⊕ Estimating user population size and usage frequency with high accuracy.
IVDR Periodic Safety Update Report for Class C & D is a new frontier for many IVD manufacturers. We assist them in:
⊕ Integrating Post-Market Performance Follow-up (PMPF) data.
⊕ Analyzing diagnostic validity and clinical performance safety.
⊕ Ensuring Class D devices meet the stringent EUDAMED submission requirements.
Ensure Your PSUR is Audit-Ready
Don’t risk your CE Certification with incomplete technical documentation. I3CGLOBAL’s expert medical writers specialize in EU MDR 2017/745 Article 86 compliance, transforming complex clinical data into high-quality Periodic Safety Update Reports.
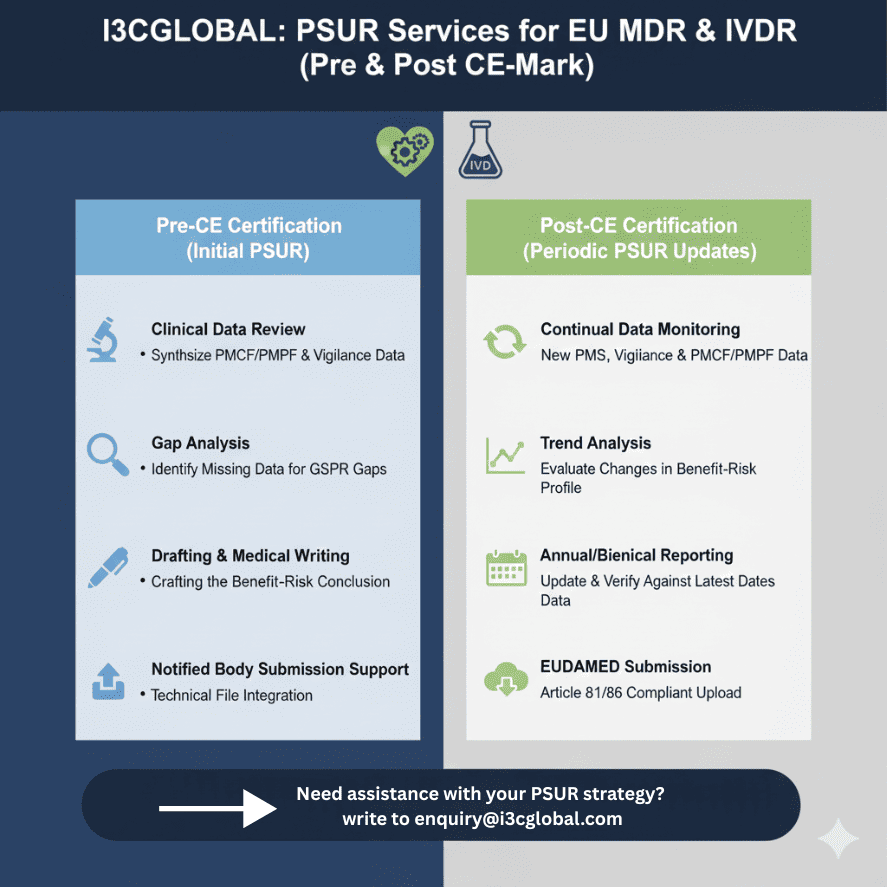
I3CGlobal's 4-Step PSUR Delivery Process
Our specialized medical writing team eliminates the complexity of Article 86 and Article 81 compliance. We don’t just fill templates; we analyze your data to build a safety narrative that satisfies Notified Body auditors.
-
We review your current PMS plan and data to ensure all variables required for a PSUR are present.
-
Our expert medical writers draft the report, focusing on the conclusions of the benefit-risk determination.
-
Every report undergoes a peer review to ensure it meets the latest MDCG guidelines.
-
We assist in the finalisation and, if required, the upload to EUDAMED or submission to your Notified Body.
- We coordinate with Authorities and Notified Body if any discrepancies or comments throughout the service tenure
Our experts have created editable Medical Device PSUR templates in MS Word that cover Procedures, Plans, and Records.
Frequently Asked Questions
When should the first PSUR be prepared?
The clock starts from the date of the CE certificate issuance under MDR/IVDR or the date the device is placed on the market.
Is a PSUR required for Class I/A/B devices?
No. Class I devices (MDR) and Class A/B devices (IVDR) require a Post-Market Surveillance Report (PMSR) instead of a PSUR. The PMSR is updated when necessary and made available to the Competent Authority upon request.
Does the PSUR need to be uploaded to EUDAMED?
For Class III devices and implantable devices (MDR), and Class D devices (IVDR), the PSUR must be submitted to the Notified Body through the EUDAMED electronic system.
When and where to conduct Periodic Safety Update Report?
As per EU MDR article 86, the Periodic Safety Update Report must be prepared by the manufacturers of moderate and high-risk devices (class IIa, IIb, and III) and must be prepared for throughout the lifetime of the device, after it has been released to the market (as it requires PMS information). It must be submitted to the Notified body or competent authority at the time of conformity assessment and is part of the technical documentation.
How should I conclude PMSR?
PMS Report (PMSR) and PSUR are two different documents as of the MDR. PMSR is for class I devices and PSUR is for moderate and high-risk devices. The conclusion of PMSR will be based on the data collected from the PMS of the class I device and the CAPAs taken for it.
The PSUR, on the other hand, must have a conclusion about the data collected from risk-benefit, PMCF, the volume of sales, usage frequency and user population information, ultimately checking the safety and performance of the devices which can be input to documentation such as CER, RMF, IFU and so on.