Quick Contact
Post Market Surveillance Report
Post market surveillance report is necessary and an obligation of the manufactures regardless for the medical device’s classification. The conformity assessment processes for acquiring a CE marking for medical devices, as defined in Annexes IX to XI, require that manufacturers create and maintain a PMSR mechanism proportional to the device’s risk class and type, but only the requirements change as per the risk class.
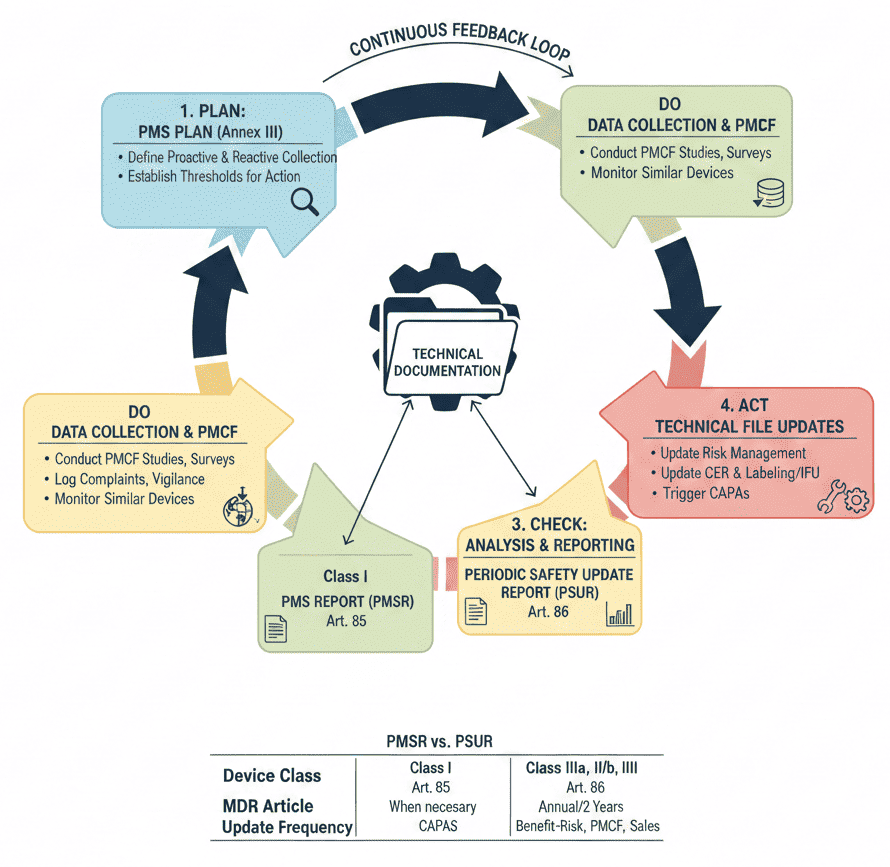
Medical device manufacturers must base their system on a PMS Plan (Article 84), which must be included in the technical documentation and must demonstrate compliance with the MDR 2017/745 PMS criteria. Annex III lays out the standards and substance of a PMS strategy, which must address the collection and use of post-market data.
Article 2, section 60 of MDR defines PMS report as a proactive and systematic process that manufacturers implement and carry out with other economic operators such as importers, distributors, authorized representatives etc.; in order to take corrective and preventive action (CAPA) in accordance with the information on medical device and their performance.
The post market surveillance report (PMS) of incidents involving medical devices allow identification of problems with the design, manufacture or use of medical devices and, ultimately, enhances patient safety. The aim of the Post Market Surveillance Report system is to actively and systematically gather, record and analyze relevant data on the quality, performance and safety of a device throughout its entire medical device lifetime.
This allows manufacturers to continuously update the risk-benefit assessment and to initiate necessary measures without delay. Manufacturers are obliged to collect and assess all information about their medical device and related devices from competitors.
PMS Report Template
Buy easy-to-edit Microsoft Word post market surveillance report template prepared by subject experts for sale. All Templates are made as per the requirements of EN ISO 13485:2016, section 8.2.1 and MEDDEV NB-MED/2.12/Rec.1 guideline
Post Market Surveillance Report is a necessity in MDR Technical Documentation
Yes, it is absolutely true. A Post Market Surveillance Report for class 1 devices and its higher risk PSUR is a mandatory component of the technical documentation under the MDR 2017/745. Notified Body or an inspection by a Competent Authority, the absence of updated PMS Report documentation Notified Bodies will flag this as a major non-conformity during technical file review and QMS audits.
The MDR is built on a “lifecycle” approach. Annex II (Technical Documentation) and Annex III (Technical Documentation on Post-Market Surveillance) explicitly state that the PMS plan and the resulting reports are essential parts of the product’s technical file.
You cannot have a complete technical documentation set without them. While a clinical trial or evaluation proves a device is safe before it hits the market, real-world usage reveals rare side effects, long-term performance issues, or “off-label” uses. The PMS Report “closes the loop” by updating the Clinical Evaluation Report with real world data.
Medical Device manufacturers must form a cross-functional post market surveillance team that will collaborate with other departments and the regulatory team to analyze methods and design a Post Market Surveillance Report.
Manufacturers may more easily identify the target demographic and anticipate hazards with the aid of consultants and regulatory specialists like I3CGlobal. In the same way, when a new technology is brought to the market, an efficient monitoring program must be created to ensure the early identification of issues.
Features of a Good PMS Report
While developing a Post Market Surveillance Report, it is essential to evaluate the product technology in connection to the manufacturer and the marketplace of similar devices. There may be a lack of awareness of the patient population and the complexity of the device state among new technology makers.
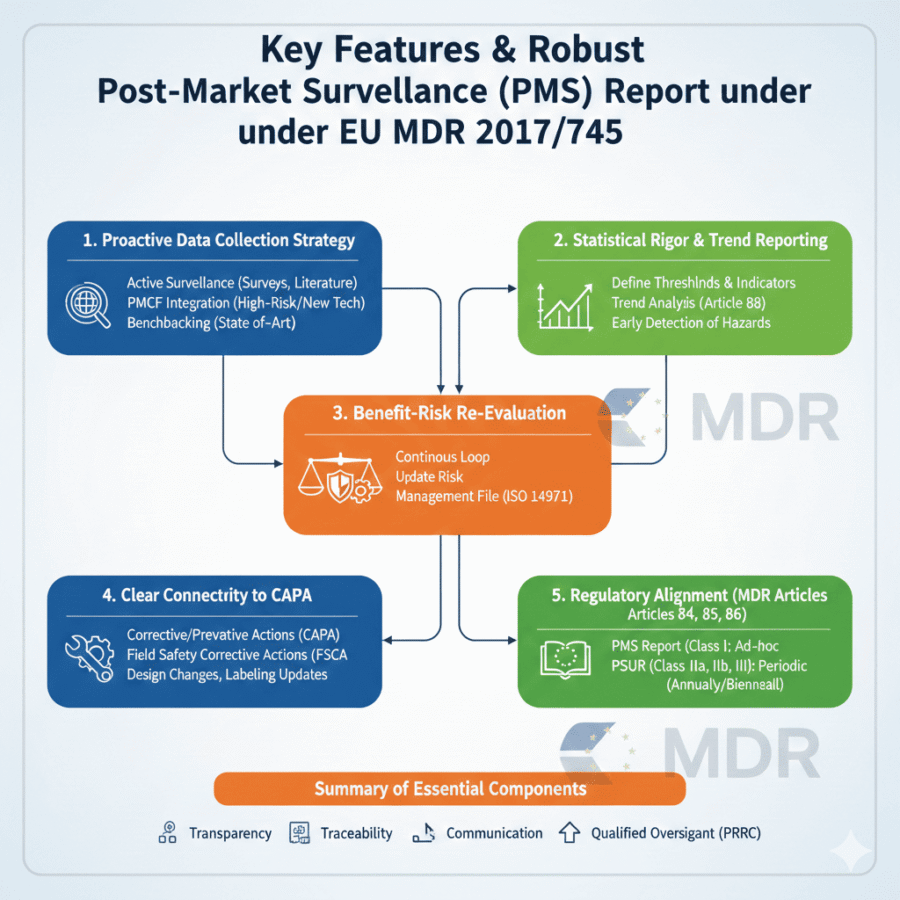
A strong Post-Market Surveillance (PMS) Report is no longer just a regulatory checkbox; under the EU MDR 2017/745, it is a dynamic document that bridges the gap between clinical expectations and real-world performance. To create a report that satisfies both Notified Bodies and safety requirements, certain features must be present.
1. Proactive Data Collection Strategy
Unlike the older MDD approach, which was often reactive (waiting for complaints), a good PMS report demonstrates a proactive system.
-
Active Surveillance: Includes user feedback, surveys, and literature reviews.
-
PMCF Integration: If your device is high-risk or uses new technology, the report must include data from your Post-Market Clinical Follow-up (PMCF) plan to validate long-term safety.
-
Benchmarking: It must compare your device’s performance against “state-of-the-art” (SOTA) or similar devices currently on the market.
2. Statistical Rigor and Trend Reporting
A key feature of a compliant report is the ability to identify shifts in device safety before they become critical failures.
-
Thresholds and Indicators: The report should define clear “trigger” points. If a complication rate exceeds a pre-defined threshold, the report must document the subsequent investigation.
-
Trend Analysis (Article 88): You must show techniques for identifying statistically significant increases in the frequency or severity of non-serious incidents.
3. Benefit-Risk Re-Evaluation
The Post Market Surveillance Report is the primary tool for updating the Benefit-Risk Analysis.
-
Continuous Loop: It should confirm whether the clinical benefits still outweigh the risks based on real-world data.
-
Risk Management Link: Any new risks identified in the field must be fed back into the Risk Management File (ISO 14971).
4. Clear Connectivity to CAPA
A “good” report doesn’t just list problems, it lists solutions for every problems and also details how the problems are mitigated. It must detail:
-
Corrective and Preventive Actions (CAPA): Any design changes, labeling updates, or manufacturing adjustments made in response to gathered data.
-
Field Safety Corrective Actions (FSCA): Documentation of any recalls or safety notices issued during the period.
5. Regulatory Alignment (MDR Articles 84, 85, & 86)
The structure of your report depends on the classification of your device:
| Feature | PMS Report (Art. 85) | PSUR (Art. 86) |
|---|---|---|
| Device Class | Class I | Class IIa, IIb, and III |
| Update Frequency | When necessary (available to authorities) | At least annually (Class IIb/III) or every 2 years (IIa) |
| Main Focus | Summary of surveillance results | Main findings, benefit-risk conclusion, sales volume |
How PMS Report is connected with ISO 13485?
The Post Market Surveillance Report should be integrated into the company’s Quality Management System (ISO 13485) and should be built together to analyze the data on quality, performance, and safety of the device throughout its entire lifecycle. It should also be allowed to draw conclusions about this data and be linked to the company’s preventive and corrective action system.
PMS could be ‘Reactive’ – responding after an event has occurred and is considered passive as they are largely data collection activities.
PMS could be ‘Proactive’ – endeavors meant to anticipate and curtail events before they occur and are considered active as they give insights and data into the real-world performance of the device
| REACTIVE Sources |
|---|
| Customer complaints |
| Literature reviews |
| User feedbacks |
| Maintenance/Service Reports |
| Failure analysis |
| In-house testing |
| Device registries |
| Social media |
| PROACTIVE Sources |
|---|
| Customer surveys |
| PMCF or other post CE clinical trials |
| Implant registries |
| Expert user groups |
Post Market Surveillance, like the ISO 14971 clause 9 requirement for post-production monitoring, should be commensurate to the risk associated with the device based on its intended usage. The lack of clarity has since been remedied with MDR, which defines PMS as one of the general duties of all producers in Article 2 (60).
Additionally, MDR has specifically called out manufacturers required to update their PMS system proactively in a comprehensive and systematic fashion in accordance with EU MDR by the personnel responsible for monitoring ensuring regulatory compliance (PRRC). This allows the manufacturers to set in place an interactive corrective action or preventive action process which is proportionate to the device type and update clinical evaluation documents.
MDR has also expressly stated that manufacturers must update their PMS system proactively in a thorough and systematic manner in line with EU MDR by people responsible for monitoring and maintaining regulatory compliance (PRRC).
This enables manufacturers to implement an interactive corrective action or preventative action approach appropriate for the device type, as well as update clinical assessment documentation. It’s crucial to remember that PMS requirements should be proportional to the danger posed by the item depending on its intended usage.
Because ISO 13485 applies to all medical devices on the market, post market surveillance strategies must be developed in conjunction with a proactive medical device PMS. Because vigilance is a QMS output, it’s useful to arrange post-market clinical data collection efforts within a device’s technical documentation.
As a result, creating a medical device vigilance system in the post-production phase to report significant device-related occurrences to authorities might help to discover previously unreported adverse product information. This evaluation may be beneficial in preventing future occurrences of situations that may have resulted in death or significant health deterioration.
Frequently Asked Questions
Explain Post Market Surveillance System
Explain how Vigilance System included in the Post Market Surveillance?
Vigilance is a result of a well-implemented ISO 13485 system, and it is thus advantageous to plan post market clinical data collection activities during the technical documentation of a device. As a result, implementing a vigilance system in the post-production phase to report major device-related occurrences to authorities might significantly aid in the discovery of previously undisclosed unfavorable product information. This assessment can help to prevent the recurrence of occurrences that could have resulted in death or major health deterioration in the future.
Why constant monitoring of medical device post market surveillance?
Constant monitoring of the device allows for early detection of hazards and production issues. It allows for the early detection of related hazards and gives the manufacturer with early feedback on device use in a real-time environment, allowing for the maintenance of good product quality. This benefits both the manufacturer and the patient.
Can I modify MEDDEV 2.7/1 Rev 3 documents to MDR Article 61 directly.
MEDDEV 2.7/1 Rev 3 & 4 is only a guidance document. Manufactures must complete documentation in line with MDR Article 61 for easy NB approval.
How can I verify Medical Device Clinical Evaluation documents are correct?
We offer a FREE tool made in line with the latest MDR. Customers can browse the page and take a trial to find the GAPS.
Is Post Market Surveillance is part of the CER documentation?
Yes, Post Market Surveillance, Post Market Clinical Follow-Up and Periodic Safety Update Reports are part of the new MDR and essential for CER documentation.