Quick Contact

Software as a Medical Device!
Software as a Medical Device refers to standalone software intended for medical purposes only, and not integrated with another device of primary use. SaMD is made usually to manage patient data, aid in decision making or support clinical decision-making. These software applications can range from simple mobile health apps to complex algorithms for diagnosing diseases or managing treatment plans.
Software as a Medical Device are subject to regulatory oversight by the FDA in the United States, MHRA in the United Kingdom or the European Commission (EC) in the European Union. Depending on the risk classification and intended use, Software medical devices requires regulatory clearance or certificates before it can be marketed and used in clinical practice.

(Listen for 9 Minutes)
Types of Software as a Medical Device
Software as a Medical Device encompasses a wide range of software applications used in healthcare for diagnostic, therapeutic, or monitoring purposes. These software devices vary in complexity and function. Here are some common types of software devices:
- Clinical Information System (CIS)
- Electronic Prescription (EP) System
- Clinical Decision Support Systems (CDSS)
- Radiology Information Systems (RIS)
- Laboratory Information System (LIS)
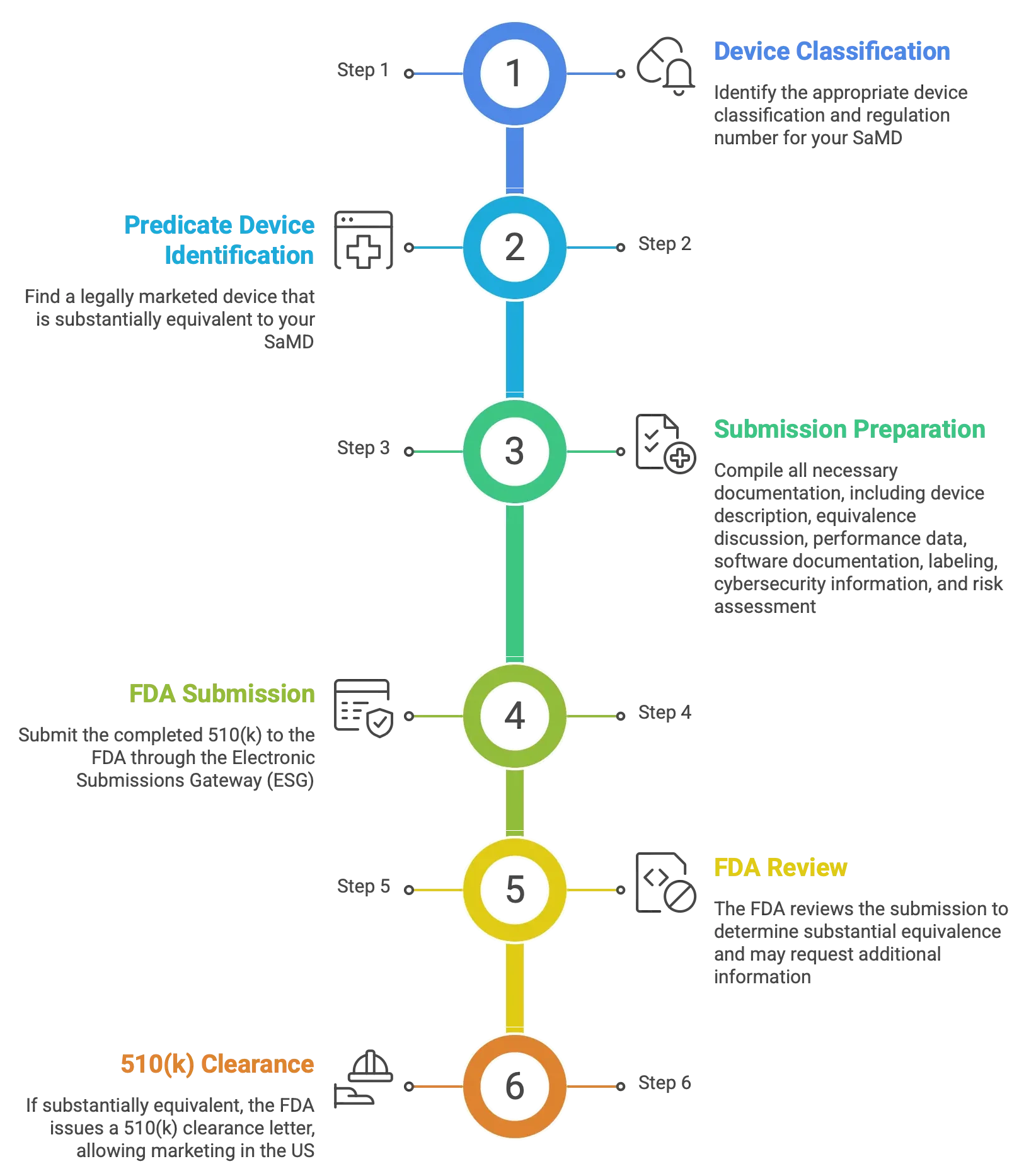
510(k) Clearance for Software as a Medical Device (SaMD)
The 510(k) pathway is a premarket submission made to the U.S. Food and Drug Administration (FDA) to demonstrate that a medical device intended for marketing in the United States is substantially equivalent to a legally marketed predicate device. A predicate device must either have an existing 510(k) clearance or be a device that was legally marketed in the U.S. prior to May 28, 1976 (pre-amendment device).
Determining if 510(k) is Required
Not all Software as a Medical Device (SaMD) products require 510(k) clearance. The FDA provides specific guidance on which software functions are considered medical devices and therefore subject to regulatory oversight.
In general, software is likely to be classified as a medical device if it is intended to diagnose, cure, mitigate, treat, or prevent disease, or if it is intended to affect the structure or function of the human body. Such software typically falls within FDA regulatory scope and may require a 510(k) submission prior to commercialisation.
Why FDA 510(k) Clearance Is Critical for SaMD Developers
Securing FDA 510(k) clearance is a critical milestone for any Software as a Medical Device developer seeking entry into the U.S. healthcare market. Unlike general-purpose software, SaMD directly influences clinical decision-making, patient outcomes, and treatment pathways, making regulatory validation essential.
FDA 510(k) clearance for SaMD demonstrates that the software is safe, reliable, and performs as intended, and that it is substantially equivalent to an already cleared predicate device. This regulatory evaluation helps mitigate patient safety risks, cybersecurity vulnerabilities, and functional failures in real-world clinical environments.
For SaMD developers, achieving 510(k) clearance builds trust with healthcare providers, hospitals, clinicians, and investors, strengthens product credibility, and enables faster adoption in regulated healthcare settings. It also protects businesses from regulatory enforcement actions, market delays, and product rejections arising from non-compliance.

Types of SaMD based on Risk
The FDA categories SaMD based on the risk it poses to patients and healthcare professionals. This risk-based approach determines the level of regulatory scrutiny required for the SaMD product. The FDA uses a four-category framework based on the IMDRF’s SaMD Risk Categorization:
-
Category I: Low Risk. SaMD that provides information to drive clinical management; however, the information is not critical to the clinical decision. Errors or inaccuracies would not be expected to cause death or serious injury.
-
Category II: Low to Moderate Risk. SaMD that provides information to drive clinical management, and the information is important to the clinical decision. Errors or inaccuracies could cause minor injury.
-
Category III: Moderate to High Risk. SaMD that provides information to drive clinical management, and the information is significant to the clinical decision. Errors or inaccuracies could cause serious injury.
-
Category IV: High Risk. SaMD that provides information to drive clinical management, and the information is critical to the clinical decision. Errors or inaccuracies could cause death or serious injury.
The risk category is determined by considering both the significance of information provided by the SaMD and the state of the healthcare situation or condition being addressed.
FDA 510k for Software as a Medical Device
Securing FDA 510k Clearance or premarket notification for a software as a medical device is a complex and stressful undertaking. The 510(k) process is a submission made to the FDA to demonstrate that a medical device is substantially equivalent to a device that is already legally marketed for the same use.
SAMD Consultants and 510k Compliance
The role of a SaMD consultants are to guide software developers through the complete process streamline the 510(k) submission process, and ultimately help get 510k clearance faster.
| Consulting Area | SaMD Consultant Role in 510(k) Compliance |
|---|---|
| 510(k) Strategy & Guidance | Guide software developers through the complete regulatory pathway and streamline the 510(k) submission process to support faster FDA clearance. |
| Substantial Equivalence Assessment | Evaluate whether the Software as a Medical Device is substantially equivalent to a predicate device by comparing intended use, technological characteristics, state-of-the-art, and performance data. |
| 510(k) Documentation Preparation | Prepare and review 510(k) submissions (Traditional or Abbreviated), including device description, indications for use, labeling, software documentation, risk analysis, version control, software verification & validation, and performance testing. |
| Gap Analysis & Remediation | Identify regulatory and technical gaps and recommend remediation actions to ensure compliance with applicable FDA requirements and standards. |
| Risk Management Support | Support the establishment of a robust risk management process for SaMD by identifying potential hazards, assessing risks, implementing controls, and documenting risk management activities. |
| FDA Communication & Review Support | Facilitate communication between the SaMD developer and the FDA during the 510(k) review process, including support for responses to FDA queries. |
| US Agent Support | Act as the US Agent to interact with the FDA on behalf of the developer, respond to information requests, and manage updates to the 510(k) file. |
Overall, the role of a SAMD Consultant is essential for small and medium-sized developers to guide the regulatory maze, streamline the 510(k) submission process, and ultimately help bring safe and effective Software as a Medical Device to market in compliance with FDA regulations.
Obtaining 510(k) clearance for SaMD requires a thorough understanding of the regulatory requirements and a well-documented submission that demonstrates substantial equivalence to a predicate device. By following the steps outlined in this document and addressing the key considerations, manufacturers can increase their chances of successfully navigating the FDA’s premarket notification process and bringing their SaMD products to market. Consulting with regulatory experts can also be beneficial in ensuring compliance with all applicable regulations.
Key Considerations for SaMD 510(k) Submissions
Do you need an email containing full details within 2 minutes? Privacy Policy>>
Role of SaMD Consultants in Achieving 510(k) Clearance
Working with experienced SaMD consultants ensures that your software’s design controls, risk management, cybersecurity controls, verification and validation activities, and regulatory documentation align with FDA expectations. Expert regulatory support significantly reduces review timelines and increases the likelihood of first-cycle 510(k) clearance.
In an evolving regulatory landscape, professional guidance is essential to transform innovative healthcare software into a fully compliant, FDA-cleared Software as a Medical Device ready for successful commercialization.
Frequently Asked Questions
What are the key components of a 510(k) submission for a software medical device?
- Device description, including software architecture and functionality.
- Risk analysis and mitigation strategies.
- Software verification and validation testing results.
- Substantial equivalence comparison to a predicate device.
- Labeling and user instructions.
What is substantial equivalence, and how do I demonstrate it?
Substantial equivalence means that the new Software as a Medical Device has the same intended use and technological characteristics as that of a predicate device or if any differences in terms of architecture, software functionality, and performance that do not raise new questions about safety and effectiveness.
What is required in the Software as a Medical Device documentation for a 510(k)?
- Software Description
- Software Requirements Specification (SRS)
- Architecture Design Chart
- Software Development Environment Description
- Verification and Validation Documents
- Risk Management File
- Traceability Matrix (linking requirements, risks, and test results)
How should I handle software modifications in a 510(k) submission?
Any modifications to the software after the initial 510k clearance must be carefully assessed to determine if a new 510(k) application is required. This is done by checking any changes on the device intended use, performance, functionality and safety. If the changes are significant, a new 510(k) application is necessary.
What is the common issues Software as a Medical Device 510(k) rejection?
- Inadequate risk management documentation.
- Insufficient software validation and verification testing.
- Failure to clearly demonstrate substantial equivalence.
- Poorly organized submission documents.
- Missing or incomplete cybersecurity considerations.
What is the role of cybersecurity in a software medical device 510(k) submission?
Cybersecurity is critical for ensuring the safety and effectiveness of software as a medical devices. The FDA requires documentation of cybersecurity measures, including threat modeling, risk assessment, and mitigation strategies. Software developers must demonstrate that the software is designed to protect against unauthorized access, data breaches, and other security threats.
Does I3CGLOBAL request a pre-submission SaMD?
Indeed, the I3CGlobal team prepares and submits pre-submission documents and questions to the FDA to receive feedback on your submission strategy. This can be especially beneficial for addressing particular concerns or questions. However, the downside is a project delay of 2-3 months.
What is expected timeline for a SAMD 510k clearance?
- How good and effectively developer implemented IEC 62304.
- Complicity of the device
- Coordination with consultants if any and the technical team involved in the project
- Pre-submission necessities
- Software (SAMD) version changes in case