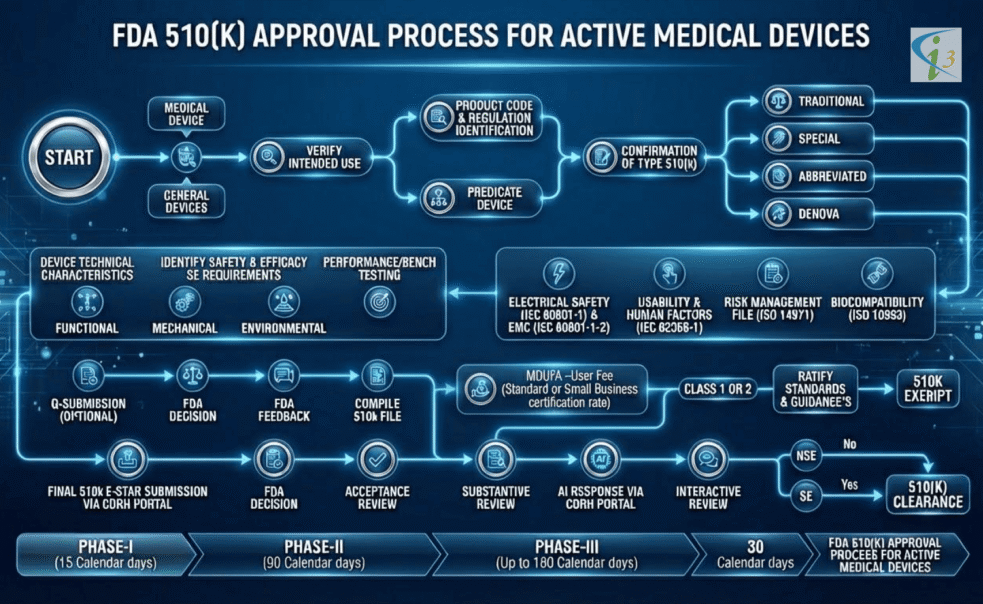
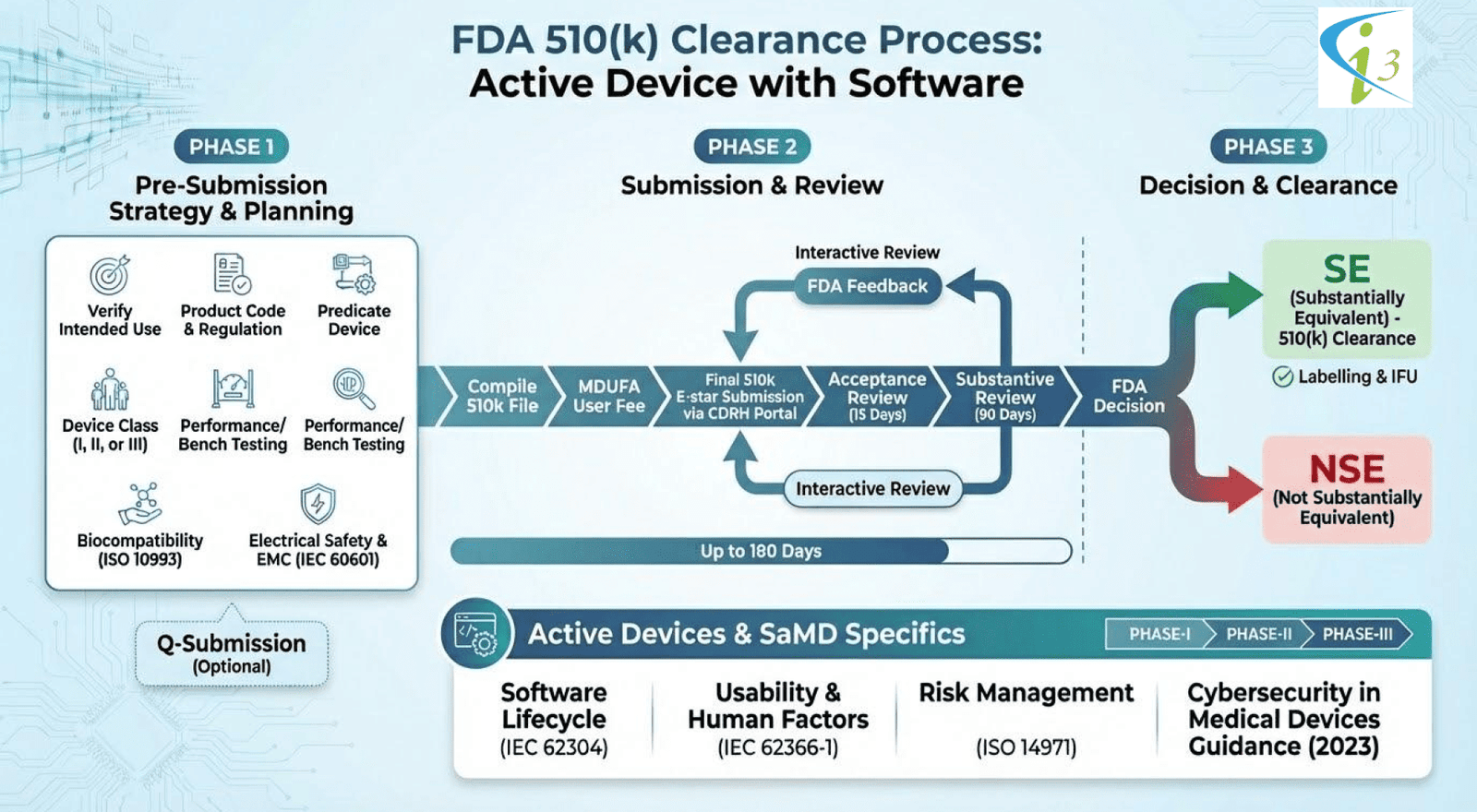
510k submission for Active Device
(With or Without a Software)
Obtaining FDA 510(k) for an active medical device, whether it includes embedded software or operates without it, requires a structured demonstration of safety, performance, and substantial equivalence to a legally marketed predicate. This page outlines the essential steps, documentation requirements, and regulatory considerations that manufacturers must follow to successfully navigate the 510(k) pathway for active devices.
Statement of Work (SOW)
|
Phase 1 – Initial Details |
|||
|
Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
| 1.1 | Intended use |
|
|
| 1.2 | Indications of Use Statement (Form 3881) |
|
|
| 1.3 | Device Description |
Send requisition for device description details through mail communication. Review all device description details shared by applicant for completeness and accuracy. Prepare and document Device Description template as per FDA premarket submission guidance requirements. |
|
| 1.4 | Predicate Device |
|
|
| 1.5 | 510(K) Summary |
|
|
|
Phase 2 – Active Device Supporting Documentation based on Indications for Use |
||||
| Document Requirements | Scope of I3CGlobal | Scope of 510(k) Applicant | ||
| 2.1 | Device Drawing | Send a requisition for a Device Drawing of an Active device and conduct final review before adding to 510k submission | Provide device drawing file from the design history file sec. 21 CFR 820.30(j) | |
| 2.2 | Design and Development of the device |
Send requisition for design development files. Review documentation and provide corrections if needed before moving to submission file |
Provide complete device drawing files and comprehensive design and development documentation of the active device with SIMD as outlined in 21 CFR 820.30, Design Controls, which covers design planning, inputs, outputs, reviews, verification, validation, transfer, changes, and the Design History File. |
|
| 2.3 | Material safety data Sheet | Send a requisition for a Material safety data sheet of crucial components of the Active device. Review and document for submission. |
Provide MSDS for all crucial components and detailed manufacturing flow chart of the active device. |
|
| 2.4 | Manufacturing Flow chart | Send a requisition for the Manufacturing Flow chart of the active device covering Software Architecture as per IEC 62304. Review and document for submission. | Provide a Manufacturing Flow chart of the hardware portion and software portion | |
| 2.5 | Proposed Labeling |
Send requisition for Primary, secondary and tertiary labels and on receipt review and document for 510(k) submission. |
Provide later revisions of Labels | |
| 2.6 | Proposed Device Information documents |
Send requisition for IFU, User Manual, promotional materials and website documentation Review all labelling materials and document for 510(k) submission |
Provide the latest approved versions of all documents including website url | |
| 2.7 | Packaging & Transportation |
Request packaging and transportation validation study plans and reports as per the latest standards identified. Review documentation and provide corrections if needed before moving to submission file |
|
|
| 2.8 | Usability and Human Factors |
Provides regulatory requirement level guidance & insights
|
Develops and executes HFE deliverables, including the HFE plan, URRA, formative and summative studies, and final HFE report. |
|
| 2.9 | Performance Testing (Bench) |
|
|
|
| 2.10 |
Electrical Device Characteristics and Intended Use Environments |
|
Provide Electrical Device characteristics and Intended Use Environments including:
|
|
| 2.11 | Risk Assessment | Drafting Risk management documents based on the information provided inline with ISO 14971
|
Implement and maintain the RMF, ensuring thorough hazard identification, mitigating risk, analysing hazards, verification of risk controls, and confirmation of RMF File.
|
|
| 2.12 | Consensus Standard | Send requisition of consensus standards applicable for Active device EMC and electrical safety
|
Provide confirmation of applicable consensus standards and explanation of deviated standards from FDA-recognized standards. | |
| 2.13 | Essential Performance and Immunity Pass/Fail Criteria | Send requisition for Essential Performance and Immunity tests study plan and reports as per FDA recognized standard for the active device. Review and document for 510(k) submission | Provide Essential Performance and Immunity tests study plan and reports as per FDA-recognized standards for active device | |
| 2.14 | Medical Device Configuration and Functions Tested | Send requisition for Medical Device Configuration and Functions Tested of the active device. Review and document for 510(k) submission | Provide Medical Device Configuration and Functions Tested of the active device which includes:
|
|
| 2.15 | Results of Safety Testing | Send requisition for safety testing plan and report as per FDA-recognized consensus standard recommended for active devices. Review and document for 510(k) submission |
Provide study plan and report |
|
|
Phase 3 – Substantial Equivalence and Executive Summary |
|||
|
Document Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
| 3.1 | Executive Summary |
|
NIL |
| 3.2 | Substantial Equivalence Discussion |
|
NIL |
|
Phase 4 – Administrative Documents |
|||
|
Document Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
| 4.1 |
Letter of Authorization
|
Prepare and provide a copy to the applicant to provide authorization for communication with the USFDA for both Q-Submission and 510(k) submission.
|
Provide a soft copy of the signed letter of authorization on the company or organization letterhead.
|
| 4.2 |
510(k) Cover Letter & Q-Submission Cover letter
|
Prepare and provide a template to the applicant covering all details required for the cover letter and share with the applicant, instructing them to use the letterhead and provide the authorized person’s signature.
|
Provide a Soft copy of signed 510(k) cover letter for 510(k)documentation.
|
| 4.3 |
Truthful and Accuracy Statement
|
Provide a template with the required content to be mentioned and document for submission.
|
The document signed by the contact person at the firm should be provided.
|
| 4.4 | MDFUSC (FDA Form (..)) |
Create a medical device user fee cover sheet and PIN. | Make payment to FDA. (Before submission of 510(k) file.) |
|
Phase 5 – Pre STAR Submission (PreSubmission/Q-Submission) |
|||
|
Document Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
| 5.1 |
Q-Submission (optional) Recommended prior to testing
|
Tracking of the review process and interacting with the US FDA reviewer throughout the review process. |
Confirm the Questions framed. Share the test plans and device details |
|
Phase 6 – eSTAR Submission (Final 510(k) Submission) |
|||
|
Document Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
|
6.1 |
eSTAR Preparation |
Populate and validate the eSTAR’s latest version template with all required sections, attachments and rationale (if any) |
Confirm and approve the Final Submission folder prior to eSTAR packaging. |
|
6.2 |
eSTAR Submission |
Verify successful submission and share FDA acknowledgment |
Provide any additional details if FDA issues RTA comments — RTA typically occurs due to user fee issues such as fee not received because of missing transaction charges or insufficient payment amount |