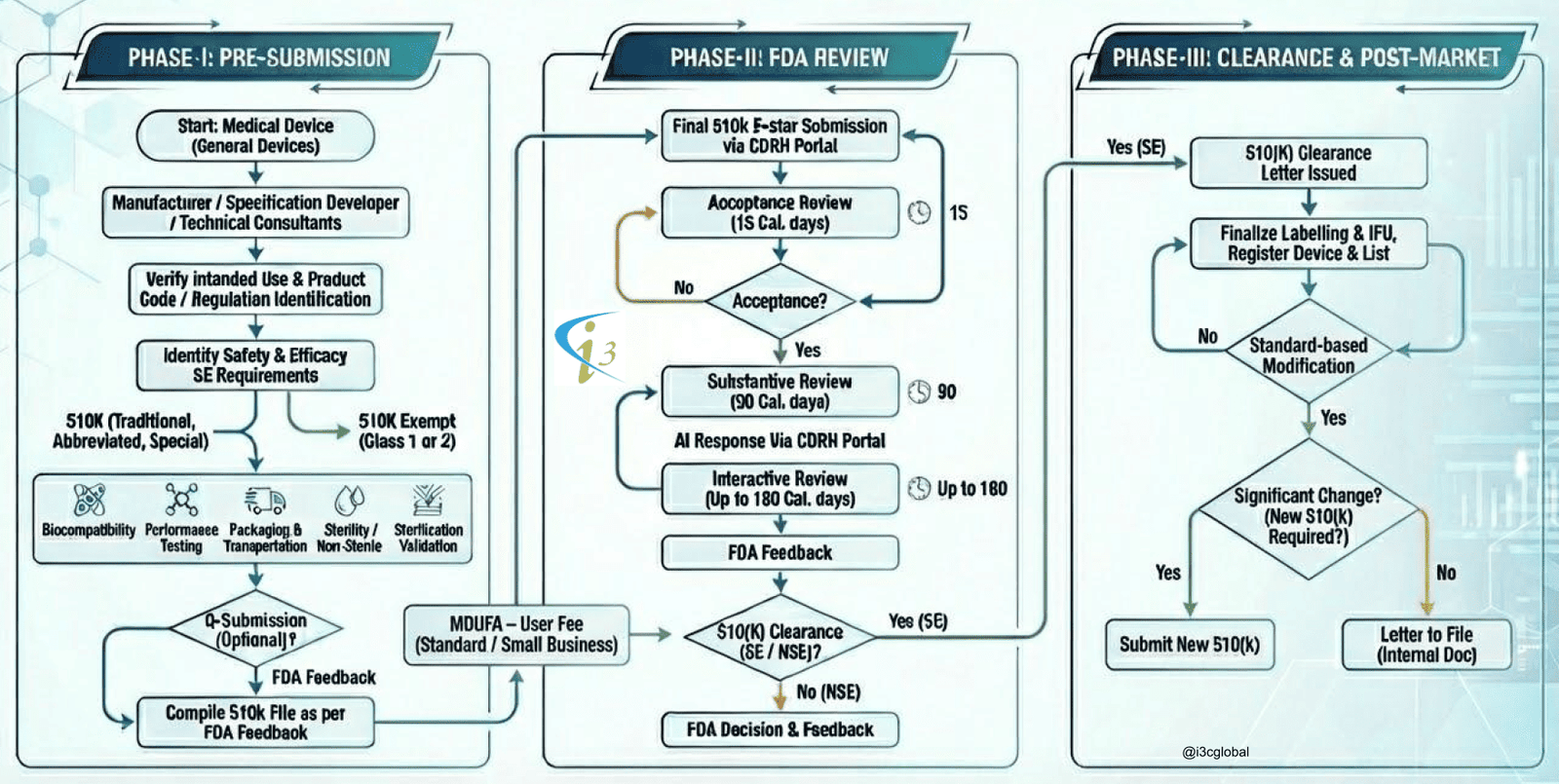
US FDA 510k Submission Process
for Non-Active Devices
A non-active medical device is any medical device that does not rely on an energy source such as electricity, batteries, or internal power to function. These devices operate purely through manual, mechanical, or gravity based actions. They do not generate or exchange energy with the body. Non-active devices are common in medical practice and many require FDA 510(k) clearance before being marketed in the United States.
510K Process Flow Diagram
(Non Active Devices)
Statement Of Work - Non Active Devices
Consultant vs. Client
|
Phase 1 – Initial Details |
|||
|
Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
| 1.1 | Intended use |
|
|
| 1.2 | Indications of Use Statement (Form 3881) |
|
|
| 1.3 | Device Description |
|
|
| 1.4 | Predicate Device |
|
|
| 1.5 | 510(K) Summary |
|
|
|
Phase 2 – Non-active Device Supporting Documentation based on Intended Use & Indications for use |
||||
| Document Requirements | Scope of I3CGlobal | Scope of 510(k) Applicant | ||
| 2.1 | Device Drawing |
|
|
|
| 2.2 | Material Safety Datasheet |
|
|
|
| 2.3 | Manufacturing Flow chart |
|
|
|
| 2.4 | Proposed Labelling |
Once agreed move the labels to the submission folder |
|
|
| 2.5 | Sterilization (If the device is sterile) |
Once agreed move the documents to the submission folder |
|
|
| 2.6 | Shelf Life |
Once agreed move the documents to the submission folder |
|
|
| 2.7 | Biocompatibility |
Once reviewed and accepted move the documents to the submission folder |
|
|
| 2.8 |
Performance Testing (Bench) (Includes Transportation and Packaging Testing along with device-specific testing based on design and mode of action) |
Once reviewed and accepted move the documents to the submission folder |
Provide justification for tests not performed that are recommended by the USFDA |
|
| 2.9 | Risk Management File (Recommended only based on the risk associated with the device not mandatory for every non-active device as per FDA) |
Once reviewed and accepted the RMF documents are moved to the submission folder |
|
|
|
Phase 3 – Substantial Equivalence and Executive Summary |
|||
|
Document Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
| 3.1 | Executive Summary |
|
NIL |
| 3.2 | Substantial Equivalence Discussion |
|
NIL |
|
Phase 4 – Administrative Documents |
|||
|
Document Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
| 4.1 | 510(k) Cover Letter |
|
|
| 4.2 | Truthful and Accuracy Statement |
|
|
| 4.3 | Declarations of conformity and Summary report |
|
|
| 4.4 | MDFUSC (FDA Form 3601) |
|
|
|
Phase 5 – Pre STAR Submission (PreSubmission/Q-Submission) |
|||
|
Document Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
| 5.1 |
Q-Submission (optional) Recommended prior to testing
|
Tracking of the review process and interacting with the US FDA reviewer throughout the review process. |
Confirm the Questions framed. Share the test plans and device details |
|
Phase 6 – eSTAR Submission (Final 510(k) Submission) |
|||
|
Document Requirements |
Scope of I3CGlobal |
Scope of 510(k) Applicant |
|
|
6.1 |
eSTAR Preparation |
Populate and validate the eSTAR’s latest version template with all required sections, attachments and rationale (if any) |
Confirm and approve the Final Submission folder prior to eSTAR packaging. |
|
6.2 |
eSTAR Submission |
Verify successful submission and share FDA acknowledgment |
Provide any additional details if FDA issues RTA comments — RTA typically occurs due to user fee issues such as fee not received because of missing transaction charges or insufficient payment amount |
