Quick Contact

Class A Sterile IVDR Classification
IVDR Class A Sterile IVDR Classification refers to devices fall under the lowest risk category for in vitro diagnostic medical devices, but with an added focus on sterility requirements. While class A devices can be self-certified by the manufacturer, Class A Sterile devices require Notified Body involvement exclusively for sterility aspects. These devices must comply with IVDR Annex I, General Safety and Performance Requirements and also maintain robust quality management systems, and provide clear technical documentation, including sterilization validation, packaging integrity, and shelf-life data. Class A sterile devices are commonly used in laboratories
Support from experienced IVDR Consultants is essential, even if the employees are experienced with IVDD
Key Requirements for Class A Sterile IVDR Certification
Class A Sterile IVD devices fall under the lowest risk category in IVDR but require Notified Body involvement for sterility aspects, unlike non-sterile Class A which is self-certifiable. To obtain CE certification under IVDR, manufacturers must demonstrate safety, performance, sterility assurance, and regulatory compliance through robust documentation and quality controls.
Technical Documentation (Annex II & III) must include the following
-
Device description & intended purpose
-
Design & manufacturing process details
-
Risk Management File (ISO 14971)
-
Biological safety & biocompatibility data (ISO 10993)
-
Sterilization validation & routine controls
-
Packaging validation (ISO 11607)
-
Shelf-life/accelerated aging studies
-
Performance evaluation as applicable
-
Evidence of validated sterilization process (ISO 11135/11137/17665 etc.)
-
Sterile barrier system integrity demonstration
-
Endotoxin/bioburden testing reports
-
Environmental controls & batch release criteria
IVDR Class A Sterile Conformity Assessment Routes
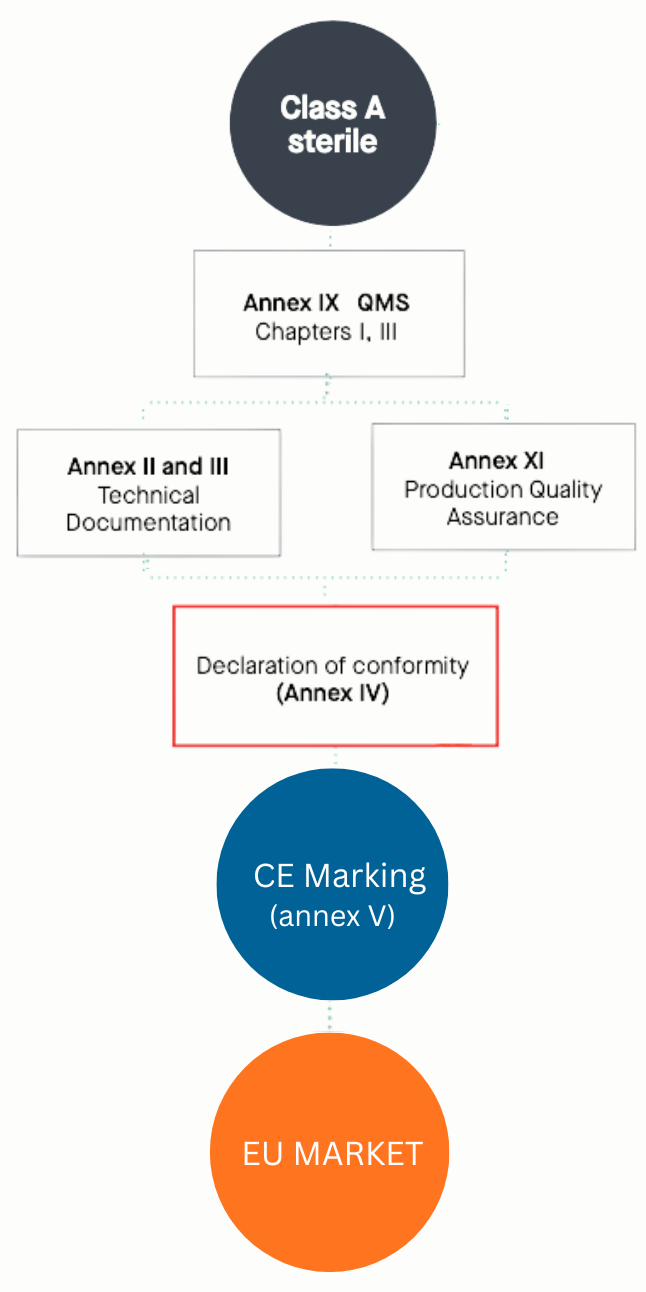
Note 1
Annex XI of IVDR (EU) 2017/746 describes the Production Quality Assurance conformity assessment route for in vitro diagnostic medical devices. Under this route, the manufacturer is required to establish and maintain complete technical documentation, however, the Notified Body’s assessment is limited to the aspects related to sterility and the associated quality management system. The manufacturer must demonstrate that controlled and validated production systems consistently ensure product quality, sterility (where applicable), performance, and regulatory compliance for each batch released to the market. This route places primary emphasis on manufacturing process, final product conformity, and quality assurance activities, rather than a full design assessment.
How to Upgrade Previously IVDD Class A Sterile Devices to IVDR Class A Sterile
Devices that were previously classified as Class A Sterile under IVDD 98/79/EC must now comply with the stricter requirements of EU IVDR 2017/746. Manufacturers need to ensure their Quality Management System (ISO 13485:2016) is fully implemented, prepare complete Technical Documentation including sterilization validation, packaging integrity, and shelf-life data, and demonstrate conformity with Annex I GSPRs. UDI assignment, EUDAMED registration, and robust Post-Market Surveillance (PMS) and vigilance systems are mandatory.
Do you need an email containing full details about IVDR Classification within 2 minutes? Share your email below: Privacy Policy>>
Frequently Asked Questions
Can I3CGLOBAL help with Class A Sterile IVDR Certification?
Yes, I3CGLOBAL provides complete consulting support including technical file preparation, sterilisation validation and documentation assistance, NB communication, labelling review, EU Representative and EUDAMED, ensuring smooth and economical approval.
What are common challenges in Class A Sterile submissions?
Gaps typically arise in sterility validation evidence, packaging validation, PMS planning, risk management traceability, and labeling compliance, which expert support can help streamline.
Is sampling applicable for Class A Sterile device during Notified Body review of Technical Documentation?
For Class A Sterile IVD devices, the Notified Body is primarily involved to assess sterility aspects only, and technical documentation sampling may apply depending on the manufacturer’s device range and similarity of sterilization processes. The NB may review one or more representative products to ensure sterilization validation, packaging integrity, and quality controls are consistent across all variants. However, if the device portfolio varies in material, process, packaging, or intended use, the NB may require full document review instead of sampling.