Quick Contact
Class B IVDR Classification
IVDR Class B IVDR classification refers to low-to-moderate risk in vitro diagnostic devices, which require mandatory Notified Body involvement. Manufacturers must demonstrate compliance with IVDR Annex I, General Safety and Performance Requirements, maintain an EN ISO 13485 compliant QMS, and prepare complete Technical Documentation under Annex II & III, including analytical/performance data, risk management, and clinical evidence where relevant.
Validation of manufacturing processes, sample stability, usability evaluation, and labelling requirements must be addressed, ensuring traceability through UDI and EUDAMED registration. Class B devices undergo conformity assessment routes such as Annex IX (QMS + Technical File Review) or Annex XI (Production Quality Assurance) depending on the manufacturer’s approach as explained in the below flow chart. These devices are widely used in clinical laboratories, hospitals, and diagnostic centres for routine testing and screening, where performance accuracy and reliability are essential.
One partner for everything IVDR Class B — regulatory guidance + technical support combined. Manufacturers, reach out to us for seamless compliance!
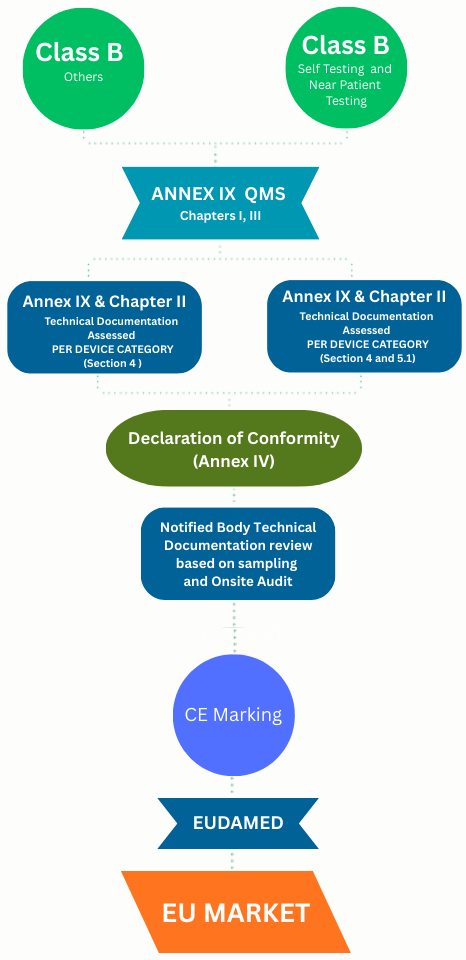
The new IVDR Class B Conformity Assessment Routes
Note 1
For Class B IVDR devices, technical assessment becomes applicable when the device is no longer self-certifiable under IVDR, a Notified Body conformity assessment route (Annex IX or XI) is selected, and the notified body application and Technical Documentation is submitted device-category wise.
Note 2
Notified Body will not review only one file, they will assess a representative device from each category to ensure all devices under that category meet the same IVDR safety and performance requirements.
Note 3
-
Section 4 of Annex IX the NB assessment of the quality management system and selection of representative sample(s)
-
Section 5.1 of Annex IX refers to technical documentation assessment for the selected device category or group
Do you need an email containing full details about IVDR Classification within 2 minutes? Share your email below: Privacy Policy>>
Transition Provisions for IVDD Self-Declared Devices Now Requiring Notified Body Involvement Under IVDR (Class B Devices)
Devices that were self-declared under the IVDD but now require a Notified Body under the IVDR, and had a valid Declaration of Conformity before May 26, 2022, are eligible for the transition extension. This applies to Class B devices moving from IVDD to IVDR classification.
To maintain market continuity, manufacturers must comply with specific transition deadlines. The Quality Management System (QMS) must be upgraded to meet IVDR requirements within the set timeframe. In addition, the manufacturer must apply to and establish a formal agreement with a Notified Body to continue placing the devices on the EU market.
Key Compliance Milestones for Class B Devices
Devices self-declared under IVDD and requiring NB involvement under IVDR are eligible for transition if DoC existed before May 26, 2022. Such devices may continue to be placed on the market or put into service until December 31, 2029 by following the below condition.
- EN ISO 13485:2016 must be upgraded to comply with IVDR by May 26, 2025.
- An application must be lodged with a Notified Body before May 26, 2027.
- A formal written agreement with the Notified Body must be signed before September 26, 2027.
Frequently Asked Questions
Can I3CGLOBAL support both regulatory documentation and product technical consultation?
Can we continue selling our Class B devices during transition?
What does ``technical assessment per device category`` mean?
It refers to the NB reviewing the Technical Documentation grouped by device category, not only for a single device.The assessment includes:
-
Review of analytical/clinical performance evidence
-
Biological safety and stability data
-
Risk management file
-
Usability and software documentation (if applicable)
-
Verification/validation data
-
GSPR compliance
-
Labelling, IFU, UDI
-
PMS/PMCF planning
So instead of checking one device file, the NB evaluates each device category chosen by the manufacturer to ensure consistency and compliance across similar devices.