Quick Contact
Class D IVDR Classification
IVDR Class D Classification refers to the highest risk category for in vitro diagnostic devices, typically those intended for detecting life-threatening, transmissible agents or for ensuring the safety of blood, organs, and tissues. Unlike lower classes, Class D devices require full Notified Body assessment, including stringent evaluation of Technical Documentation, performance verification, and ongoing batch release verification by an EU Reference Laboratory (where applicable). These devices must fully comply with IVDR Annex I GSPR and strong Quality Management System, and provide extensive clinical and analytical performance evidence. Class D devices are critical to public health protection and are widely used in blood screening, infectious disease diagnostics, and high-risk pathogen detection.
I3CGLOBAL offers expert regulatory and product technical consultation for Class D IVDR CE Certification from performance evaluation, technical file preparation, to Notified Body submission and coordination, all delivered efficiently and economically. Talk to regulatory experts and product experts soon.
IVDR Class D Conformity Assessment Routes
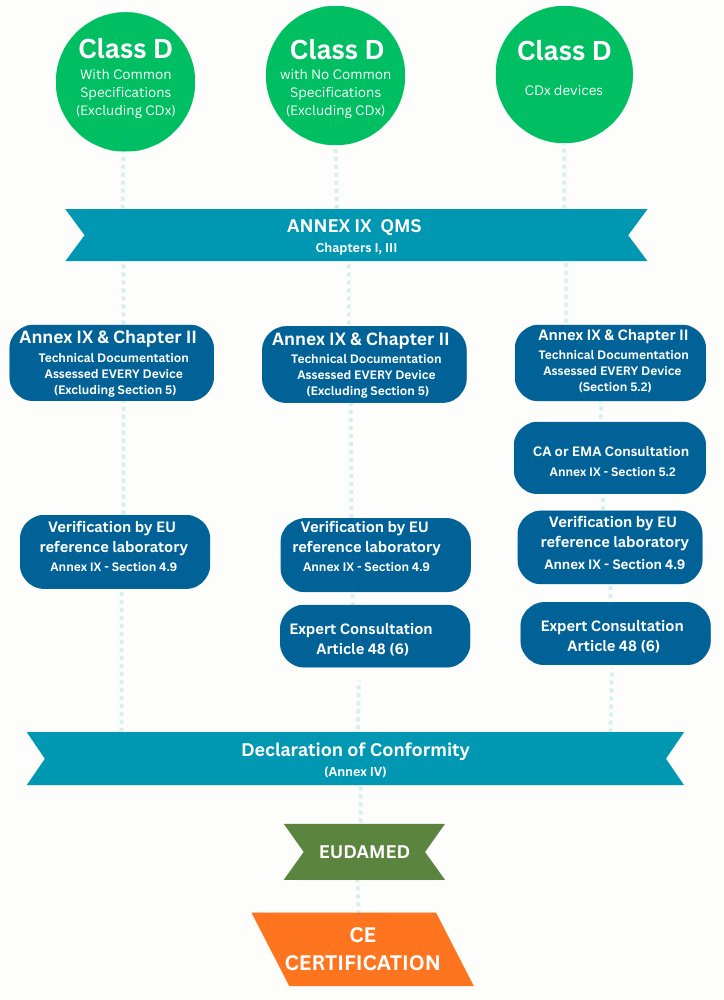
Applicable requirement's to be followed by class D manufactures throughout the certification cycle
- Performance Evaluation Report updates (Annex XIII – Part A, Section 1.3.2 and Article 56): Updated at least annually. Notified Body will provide it to the expert panel as needed. Notified Body to review at the time of PSUR reviews or substantial change reviews
- Post Market Performance Follow-up (PMPF) updates Evaluation Report (Article 56 and Annex XIII, Part B: Updated as per manufacturer’s PMS, PMPF plans. Notified Body to review at the time of PSUR reviews or substantial change reviews
- Post Market Surveillance (PMS) Report (Article 80) : Post-market surveillance will be captured in the Periodic Safety Update Report
- Periodic Safety Update Report (PSUR) (Article 81): PSUR update required at least annually. Submitted to the Notified Body via EUDAMED for Notified Body review
- Summary of Safety and Performance (Article 29): Updated as soon as possible, where significant change.
Do you need an email containing full details about IVDR Classification within 2 minutes? Share your email below: Privacy Policy>>
Frequently Asked Questions
What additional requirements apply to Class D devices compared to other classes?
Class D submissions require comprehensive analytical and clinical performance data, risk management justification, batch verification (if applicable), and ongoing post-market performance follow-up. The process is more exhaustive than Class B/C, with deeper scrutiny on clinical evidence, reproducibility, traceability, and public health safety.
What is the Expert Consultation Process for Class D IVDs under Article 48(6)?
Class D expert consultation as per Article 48(6) refers to the mandatory scientific opinion process where, for certain high-risk Class D IVDs, the Notified Body must consult an EU Reference Laboratory (EURL) or an Expert Panel to review the device’s performance evaluation and clinical evidence before the certificate is issued. Under this requirement, the Notified Body submits the manufacturer’s performance evaluation report, analytical/clinical data, and intended use to the expert body for assessment. The experts may provide comments, request additional evidence, or recommend further verification studies to ensure the device delivers accurate and reliable results for critical pathogens. The final CE marking decision can only proceed after these scientific opinions are considered, making this consultation an added safety layer for Class D devices with major public health impact.
What is CA or EMA Consultation for IVD Class D?
CA or EMA consultation for Class D IVDs is an additional scientific review step required during the conformity assessment of high-risk devices. For certain Class D tests, especially those used to detect serious transmissible agents or life-threatening diseases, the Notified Body must seek a scientific opinion from the Competent Authority (CA) or the European Medicines Agency (EMA). This step involves reviewing the device’s performance evaluation, clinical evidence, and benefit-risk justification to ensure public health safety. The feedback provided by CA/EMA must be addressed before final CE marking, making this consultation a critical layer of oversight in the certification of Class D IVDs.
