Quick Contact

Medical Device Single Audit Program (MDSAP)
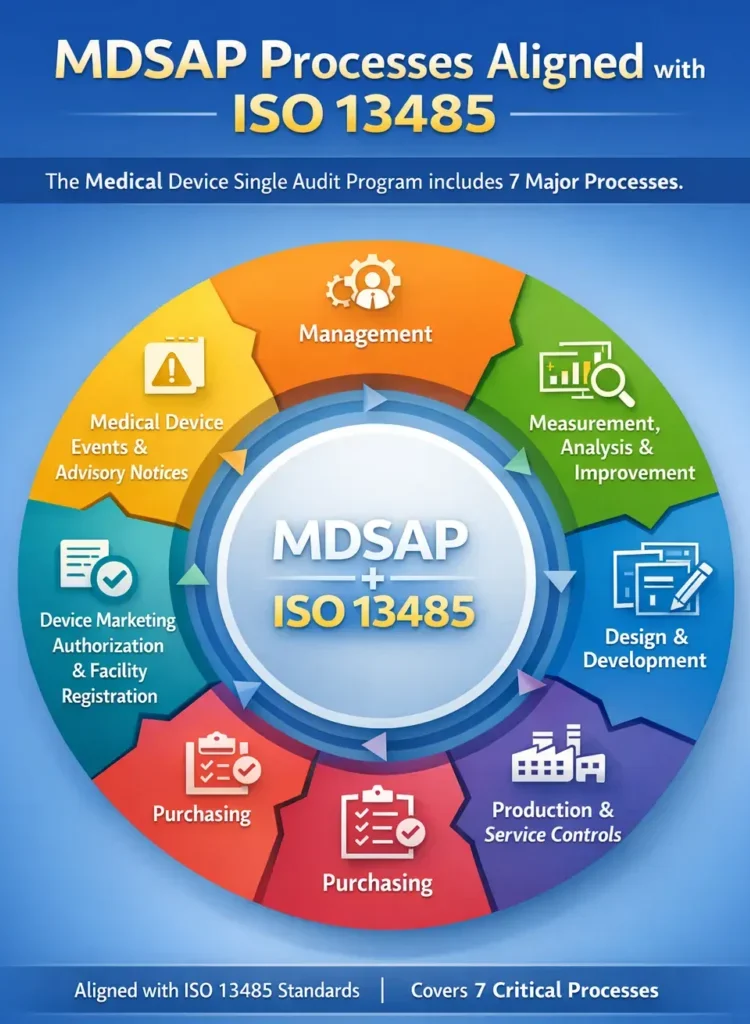
MDSAP stands for Medical Device Single Audit Program. It is an international audit program that allows medical device manufacturers to undergo one regulatory audit that is accepted by multiple regulatory authorities instead of separate audits for each country. The Regulatory Authorities Participating in MDSAP programme are the following regulators
🇺🇸 United States – FDA
🇨🇦 Canada – Health Canada (mandatory for selling medical devices in Canada)
🇦🇺 Australia – TGA
🇯🇵 Japan – PMDA / MHLW
🇧🇷 Brazil – ANVISA
Benefits of MDSAP Certification
| Benefit Area | Description |
|---|---|
| Single Audit | One audit accepted by FDA, Health Canada, TGA, PMDA/MHLW, and ANVISA. |
| Canada Market Access | MDSAP certification is mandatory to sell medical devices in Canada. |
| Reduced Audit Burden | Eliminates multiple regulatory audits, saving time and costs. |
| Regulatory Compliance | Covers ISO 13485 plus country-specific regulatory requirements. |
| QMS Improvement | Strengthens risk management, CAPA, and post-market surveillance. |
| Regulatory Credibility | Demonstrates regulatory maturity and builds confidence with authorities. |
| Faster Market Access | Reduces likelihood of additional inspections by regulators. |
| Post-Market Compliance | Enhances vigilance, adverse event reporting, and advisory notices. |
“Your Trusted Partner for MDSAP Success.”
I3CGlobal helps medical device manufacturers achieve MDSAP implementation and certification with confidence. Our consulting approach simplifies complex internal system issues, reduces audit risk, and accelerates access to key international markets. From readiness assessment to certification and beyond, we turn compliance into a strategic business advantage.
Role of I3CGlobal MDSAP Consultants
| Service Area | How I3CGlobal MDSAP Consultants Supports You |
|---|---|
| MDSAP Gap Analysis & Readiness Assessment | Evaluation of the existing QMS against ISO 13485:2016 and MDSAP country-specific regulatory requirements to identify gaps and define a clear compliance roadmap. |
| QMS Development & Alignment | Design, update, and alignment of the Quality Management System to fully meet MDSAP’s seven audit processes with strong documentation and effective implementation. |
| Regulatory Strategy & Market Focus | Alignment of the MDSAP program with target markets (USA, Canada, Australia, Japan, Brazil) and business objectives to maximize compliance ROI. |
| Training & Process Implementation | Delivery of practical, role-based training and hands-on implementation support to ensure audit readiness and sustained compliance. |
| Internal Audits & Mock MDSAP Audits | Execution of mock audits based on the official MDSAP audit model to prepare teams for real-world auditor expectations. |
| CAPA & Risk Management Support | Support for root cause analysis, CAPA development, and effective closure to strengthen compliance and audit outcomes. |
| Audit Support & Coordination | Real-time support during certification audits, including audit coordination and regulatory response management. |
| Post-Certification & Surveillance Support | Ongoing support for regulatory maintenance, surveillance audits, and continuous improvement after certification. |
MDSAP Consultation Fees
- Onsite GAP Assessment: 1000 USD per man-day + travel and accomodation
- Offsite GAP Assessment: 500 USD per man-day
- Guidance and support for fixing GAPS: 300 USD/Man-day (Offsite)
- Internal Audit: 600 USD/Man-day ( Offsite) and 1000 USD (Onsite) plus travel and accommodation
