Quick Contact

Risk Benefit Analysis Medical Devices
Risk Benefit Analysis of Medical Devices is tough and interesting. Medical devices are developed for certain medical purposes, means they have certain benefits. So, we should compare the benefits with the risks, to determine the acceptability of risks of a particular medical device.
There is a saying, “A bitter pill to swallow”, which means that we have to accept it even though it is very unpleasant. Similar way, the products which we are used for the treatment of patients having some side effects or harms, but we should use them to save the lives.
If we came to know that there is a probability of occurrence of harm, associated with the use of particular medicine or device, then we should estimate the severity of that harm. We generally called it as “Risk”. There are different levels of severities for different types of harms for a particular medical device, based on its intended use. It means there are different levels of risks associated with a device and we should estimate each risk for its acceptability to use on humans.
Role of Consultants in performing Risk Benefit Analysis
Benefit Risk Analysis plays a crucial role in the approval of medical devices, hence the organizations having a team of specialists and consultants to analyze the benefits over the risks for a medical device.
The specialists and/or consultants having wide knowledge of medical devices design, regulations are capable of comparing the benefits over the risks by providing the proper justifications. At the same they can guide the organization for any modifications in the device, if required, after the risk benefit analysis.
As the benefits estimated by quantitatively, and the risks by probability, hence the comparison requires well established methodologies.
Significance of Risk Benefit Analysis in MDR / IVDR
Risk benefit analysis helps us to estimate the severity of the harms associated with a medical device during the design and development phase. Based on that manufacturer can modify the design, change the materials, or provide more controls for the safety during its use.
If the control measures applied to the medical device doesn’t reduce its significance, those risks are considered as the Residual Risks. Risk-Benefit Analysis is done on those residual risks for the determination of its Risk-benefit ratio and its acceptability. The technical documentation as per the Annex II of MDR shall contain this information as per the requirement in the Section 1 and 8 of the Annex I GSPR.
Hence the Risk Benefit Analysis is performed during following stages of CE Marking!
- As part of risk management throughout the life cycle of the Medical Device, all risks should be reduced as far as possible without affecting the risk-benefit ratio.
- Clinical evaluation should contain the clinical evidence for the risk- benefit analysis.
- Data gathered during the Post market surveillance to update the Risk- Benefit determination and its continuous reassessment.
- PSUR shall be updated with the conclusion of the Risk-Benefit determination.
- The non serious incidents or undesirable side effects should be analyzed for risk- benefit and that have significant impact should be trend reported.
Risk Benefit Analysis should be included in the risk management file and overall acceptability of the Medical Device assessment for the CE Marking is based on the risk benefit analysis. Risk Benefit Analysis can be performed by following the standard EN ISO 14971:2019 and also the guidance on clinical evaluation MEDDEV 2.7/1 Rev 4
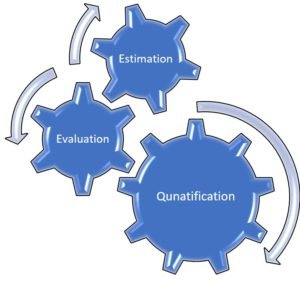
Steps involved in Risk Benefit Analysis
To carry out a risk-benefit analysis, we should follow the three steps for both the Risks and Benefits,
Estimation of Risk Benefit Analysis

The benefits of a medical device are related to its indented purpose. Benefit can be estimated from knowledge of such things as:
- Performance expected during its clinical use
- Clinical outcome from that performance
- Other treatment options
- Target groups
Quantification of Benefits

Benefits are quantified by considering the target patient population and duration of effect.
- Target patient population
- The benefits experienced only by a small proportion of patients in the target population or occur frequently in patients throughout the target population.
- Large benefit, even if experienced by a small population, considered to be significant enough to outweigh risks, and in the same way, a small benefit may not, unless it is experienced by a large population of subjects.
- The duration of effect
- The duration of a treatment’s effect
- The age of patients
Estimation of Clinical Risks
During the risk management process, we identify the risks associated with the device and how such risks have been addressed. The clinical evaluation is expected to address the significance of any risks that remain after design risk mitigation strategies that have been employed by the manufacturer during the manufacturing process.
Post Marketing Surveillance reports should also be considered by the manufacturer, whether they are device related or not, which include the details of the device’s regulatory status, actions undertaken during the reporting period and tabulation of incidents, particularly serious adverse events, including deaths.
To demonstrate the extent of the probable risks, the following factors should be addressed
- Device-related serious adverse events/ incidents
- Device-related non-serious/ non-reportable harmful events
- Procedure-related incidents
- Probability of a harmful event
- Duration of harmful events
- Risk from false-positive or false-negative results for diagnostic medical devices
- Totality of the harmful events associated with the device
- Any clinical data that identifies hazards not previously considered in the risk management documentation, outlining any additional mitigation required.
Evaluation of Clinical Risks
- Intensity of the risk
- Life threatening or not
- Medical necessity
Quantification of Risks
Risks are quantified by considering the following,
- Recurrence of adverse events
- Any other harmful affects
- Patient Population
Acceptance of Risks
Residual Risks associated with any device accepted if the benefits outweigh those risks. The manufacturer should provide sufficient clinical data to the health authorities for the review. Then the reviewers have the final call for the approval, after the Risk Benefit analysis.
Requirements for Risk Benefit Analysis
The following are mainly considered for risk benefit analysis,
- Biocompatibility data and the Benchmark Test Reports
- Clinical data of similar devices
- Post Marketing Surveillance data
