Quick Contact
Class D IVDR Classification
This webpage provides an overview of the classification of software used in in-vitro diagnostics (IVDs) under the In Vitro Diagnostic Regulation (IVDR). The classification ranges from Class A (low risk) to Class D (high risk), depending on the potential impact of the diagnostic information provided by the software on patient health and public health. Understanding these classifications is crucial for manufacturers to comply with regulatory requirements and ensure the safety and performance of their IVD software.
IVDR Classification Rules for Software
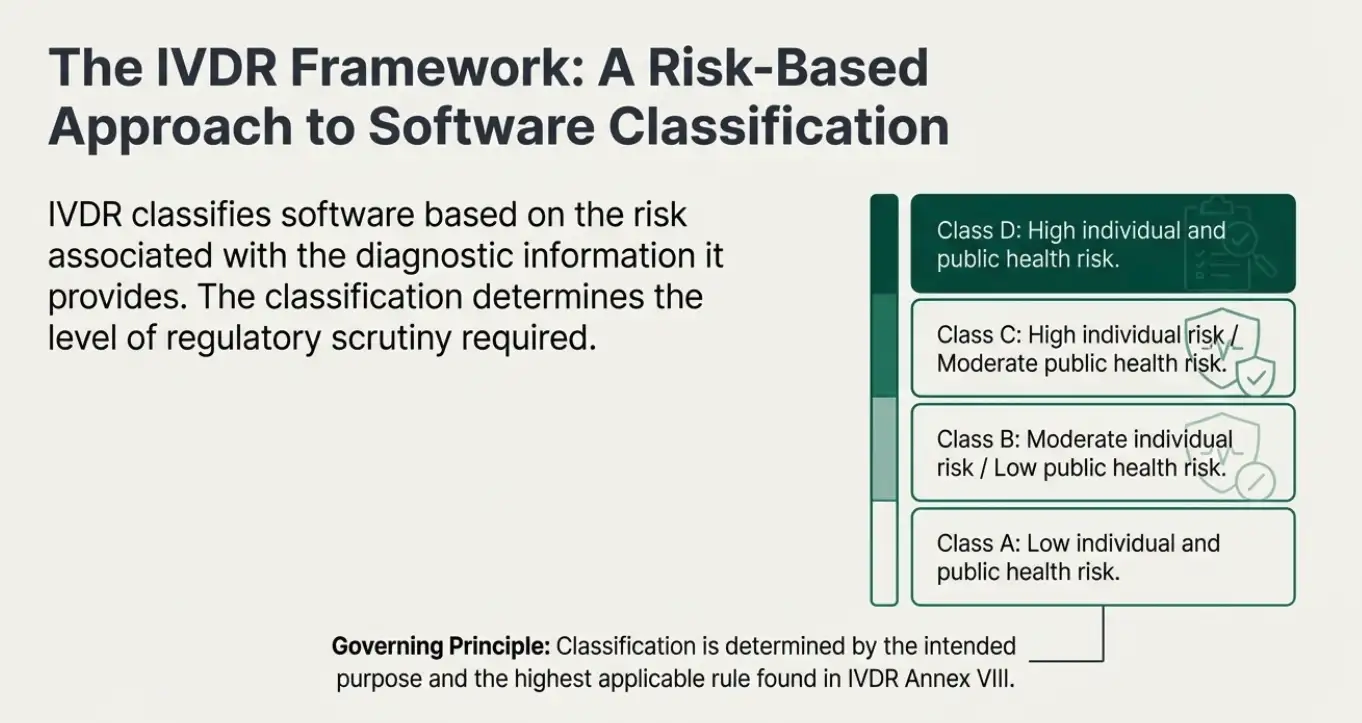
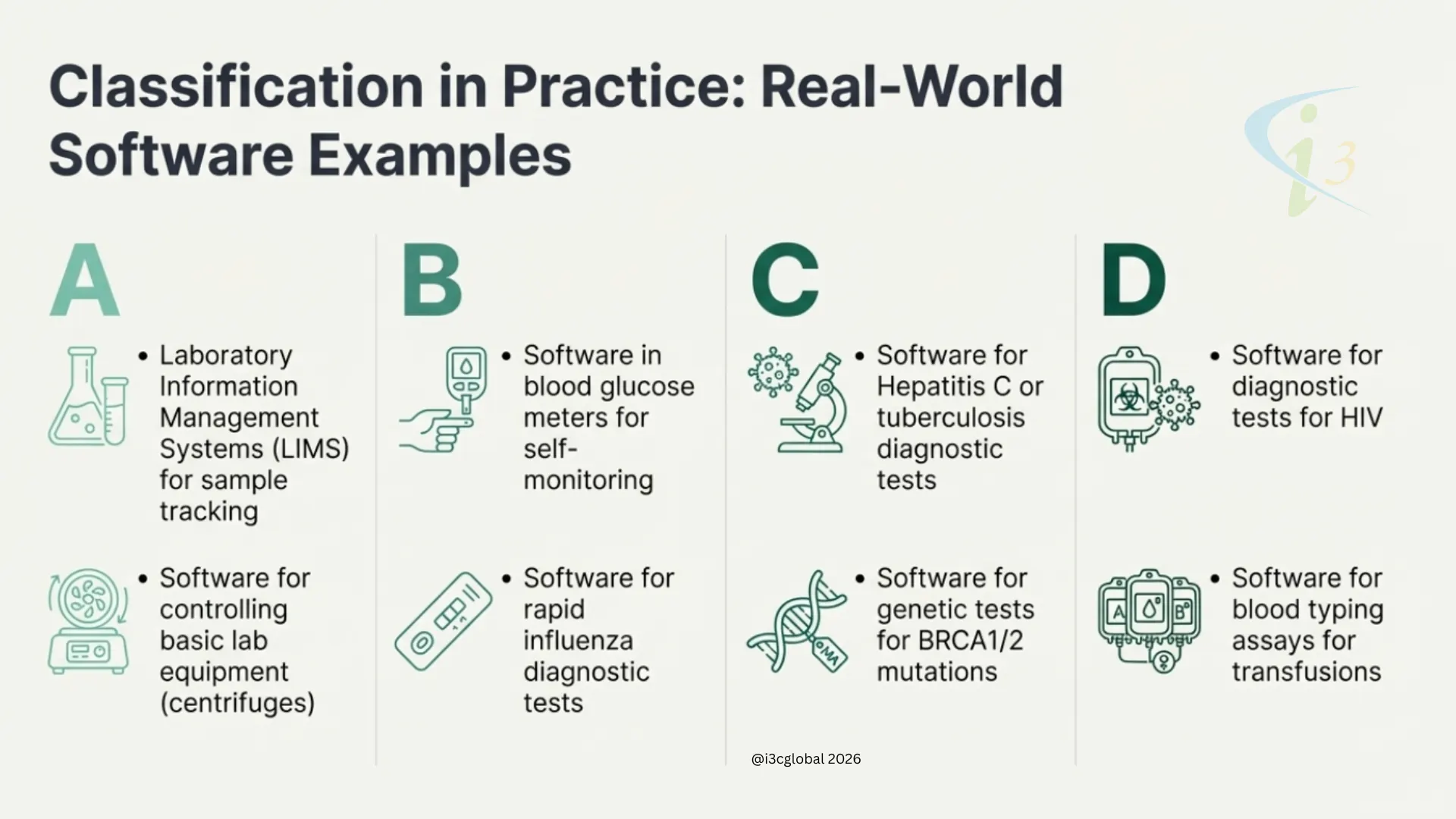
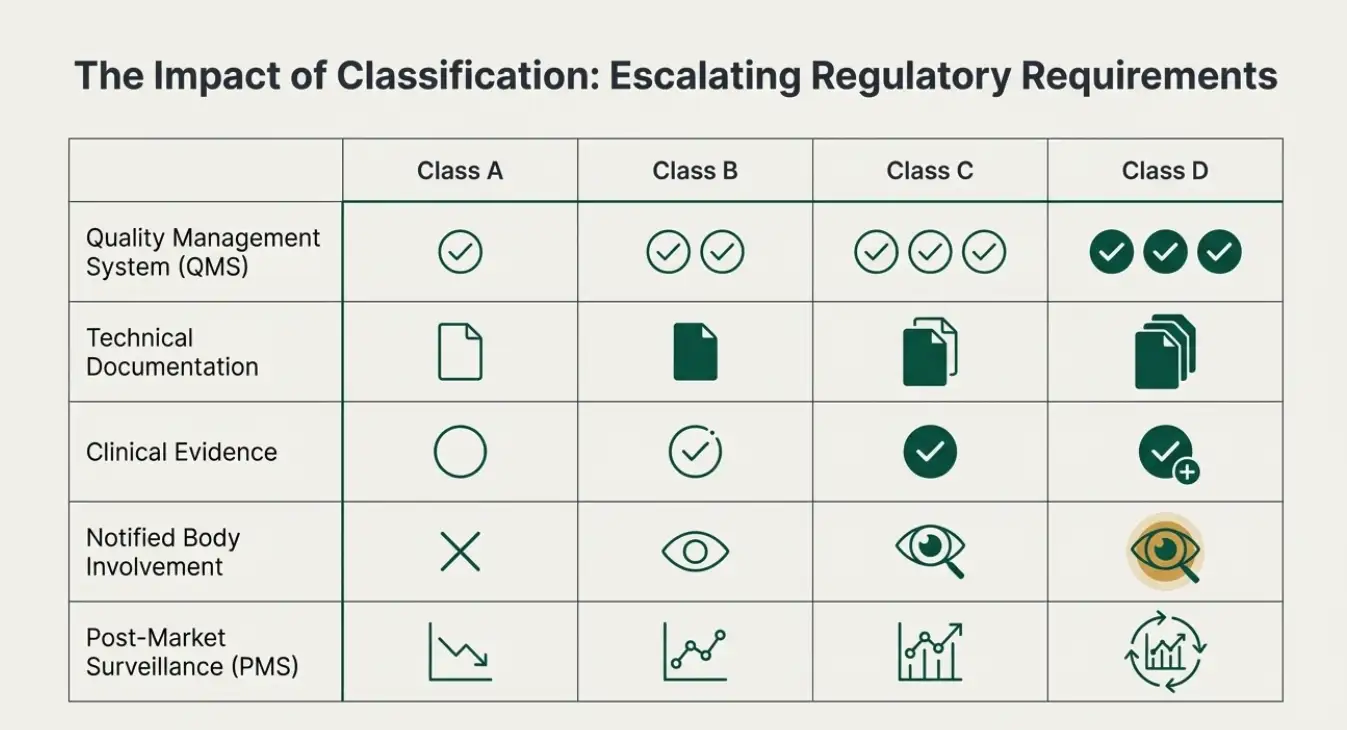
The IVDR provides a set of classification rules to determine the appropriate risk class for IVD devices, including software. These rules are outlined in Annex VIII of the IVDR. The classification depends on the intended purpose of the IVD, its risks, and the impact of its results on clinical management. Software is classified based on the highest applicable rule. Here’s a breakdown of the classification rules relevant to software in the below table
| Classification | Software Intended Use & Description |
|---|---|
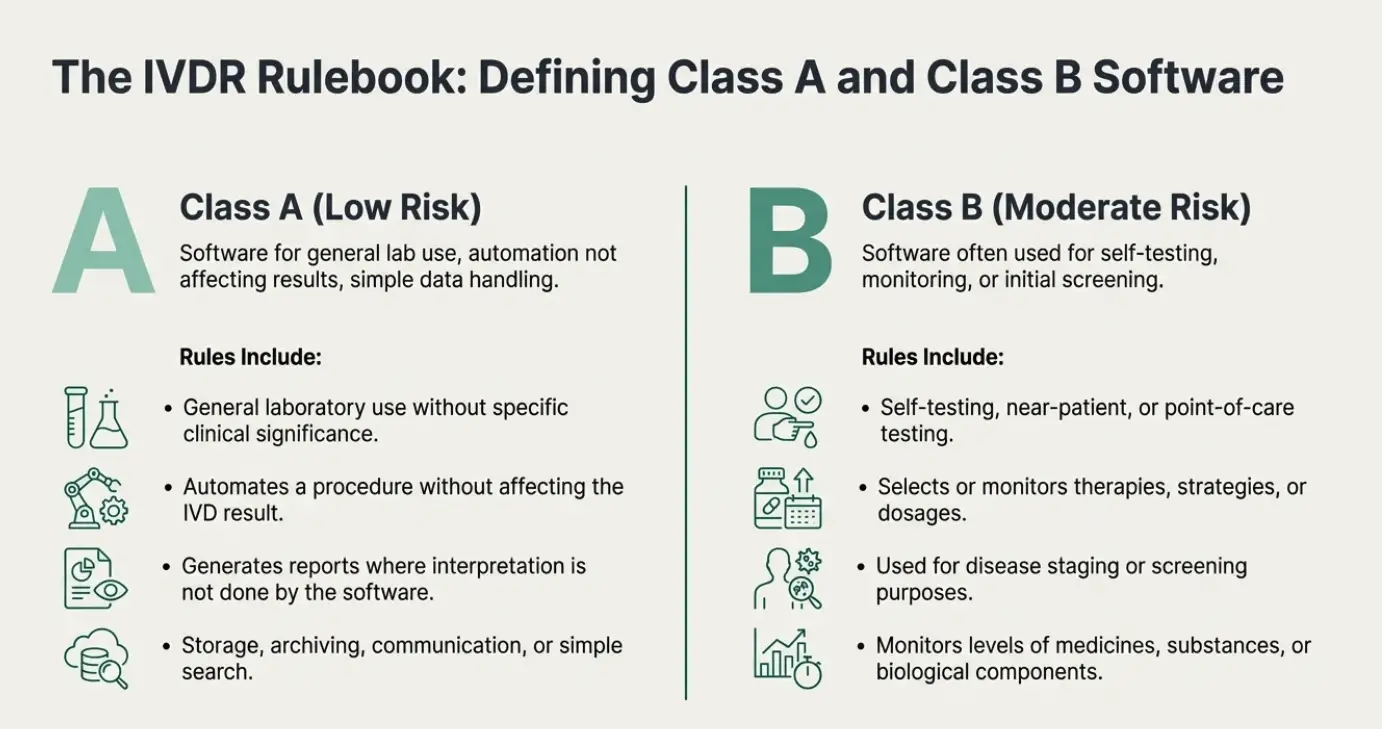
| Class A |
|
| Class B |
|
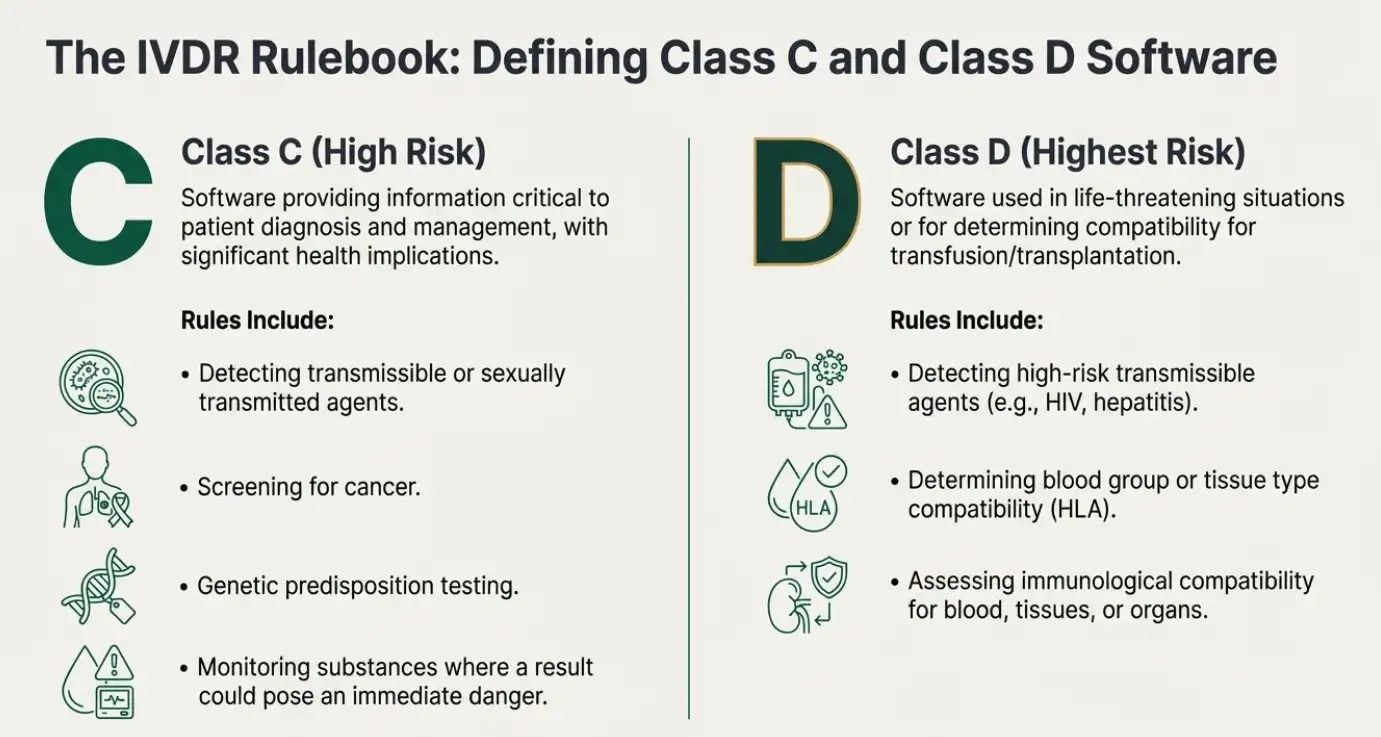
| Class C |
|
| Class D |
|
Is Your IVD Software Classification Built on Solid Ground?
Navigating the IVDR landscape is complex—from defining intended purpose to identifying the highest risk rules for SaMD. Don’t let classification hurdles stall your path to market. At I3CGlobal, we transform regulatory complexity into clinical confidence. As a leading provider for IVDR CE Marking, we ensure your software is accurately classified, future-proofed against updates, and ready for global success.
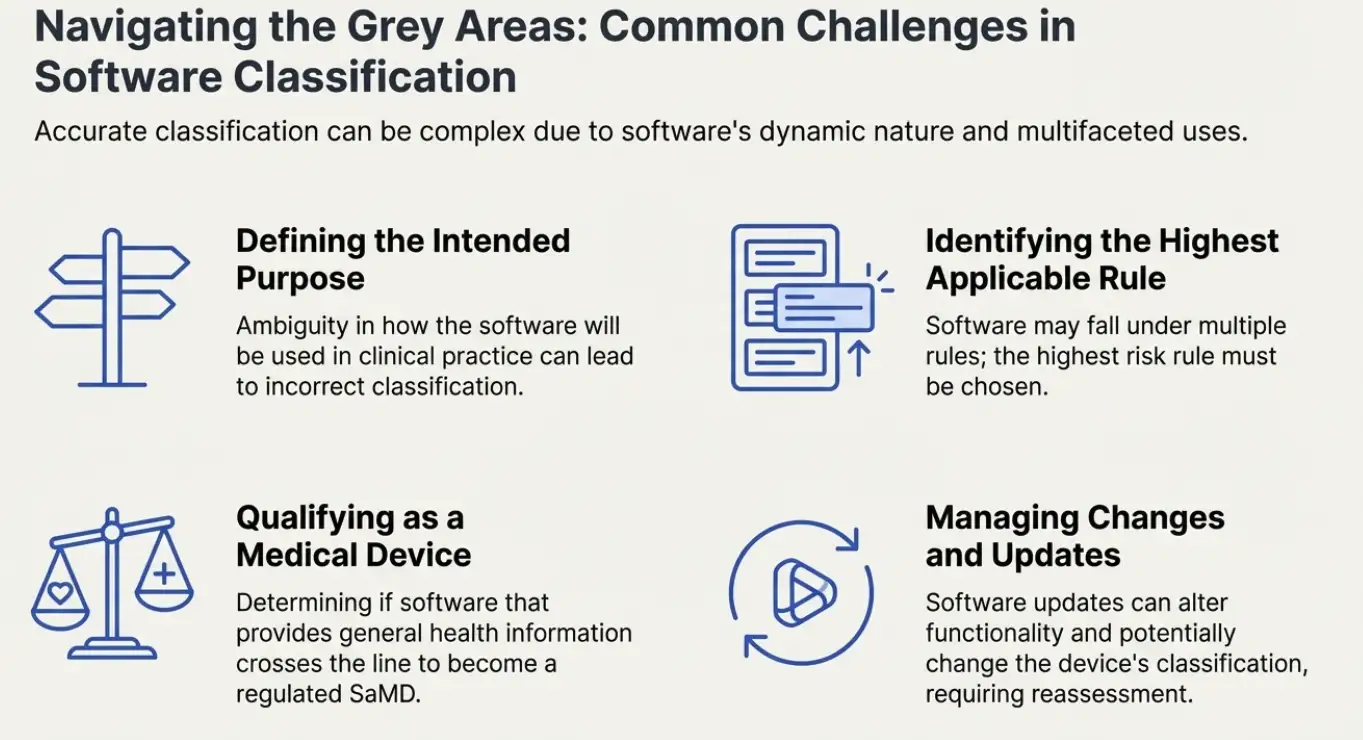
Challenges in Software Classification
Classifying software under the IVDR can be challenging due to the complexity of software functionality and the potential for multiple intended uses. Some common challenges include:
-
Determining the Intended Purpose: Clearly defining the intended purpose of the software is crucial for accurate classification. This requires a thorough understanding of how the software will be used in clinical practice.
-
Identifying the Highest Applicable Rule: Software may fall under multiple classification rules. Manufacturers must identify the highest applicable rule to ensure the software is classified correctly.
-
Software as a Medical Device (SaMD): Determining whether software qualifies as a medical device can be challenging, particularly for software that provides general health information or lifestyle advice.
-
Software Changes and Updates: Changes and updates to software may affect its classification. Manufacturers must have a process in place to assess the impact of changes on the classification of the software.
The IVDR classification of software used in in-vitro diagnostics is a critical aspect of ensuring the safety and performance of these devices. Manufacturers must carefully consider the intended purpose of their software and apply the classification rules appropriately. Understanding the impact of classification on regulatory requirements is essential for compliance and successful market access. By adhering to the IVDR classification rules, manufacturers can contribute to the safety and well-being of patients and the public.
Do you need an email containing full details about IVDR Classification within 2 minutes? Share your email below: Privacy Policy>>
Frequently Asked Questions
What additional requirements apply to Class D devices compared to other classes?
Class D submissions require comprehensive analytical and clinical performance data, risk management justification, batch verification (if applicable), and ongoing post-market performance follow-up. The process is more exhaustive than Class B/C, with deeper scrutiny on clinical evidence, reproducibility, traceability, and public health safety.
What is the Expert Consultation Process for Class D IVDs under Article 48(6)?
Class D expert consultation as per Article 48(6) refers to the mandatory scientific opinion process where, for certain high-risk Class D IVDs, the Notified Body must consult an EU Reference Laboratory (EURL) or an Expert Panel to review the device’s performance evaluation and clinical evidence before the certificate is issued. Under this requirement, the Notified Body submits the manufacturer’s performance evaluation report, analytical/clinical data, and intended use to the expert body for assessment. The experts may provide comments, request additional evidence, or recommend further verification studies to ensure the device delivers accurate and reliable results for critical pathogens. The final CE marking decision can only proceed after these scientific opinions are considered, making this consultation an added safety layer for Class D devices with major public health impact.
What is CA or EMA Consultation for IVD Class D?
CA or EMA consultation for Class D IVDs is an additional scientific review step required during the conformity assessment of high-risk devices. For certain Class D tests, especially those used to detect serious transmissible agents or life-threatening diseases, the Notified Body must seek a scientific opinion from the Competent Authority (CA) or the European Medicines Agency (EMA). This step involves reviewing the device’s performance evaluation, clinical evidence, and benefit-risk justification to ensure public health safety. The feedback provided by CA/EMA must be addressed before final CE marking, making this consultation a critical layer of oversight in the certification of Class D IVDs.