In vitro diagnostic (IVD) devices play a crucial role in modern healthcare by aiding in the detection, diagnosis, and management of a range of diseases. The U.S. Food and Drug Administration (USFDA) regulates these devices to ensure their safety and efficacy. IVD devices can be classified into two groups based on intended use, distribution, and regulatory oversight: IVD Kits and IVD Non Kits. Each group follows a distinct regulatory pathway, testing standards, and approval process.
Definition and Key Differences Between IVD Kits and IVD Non Kits
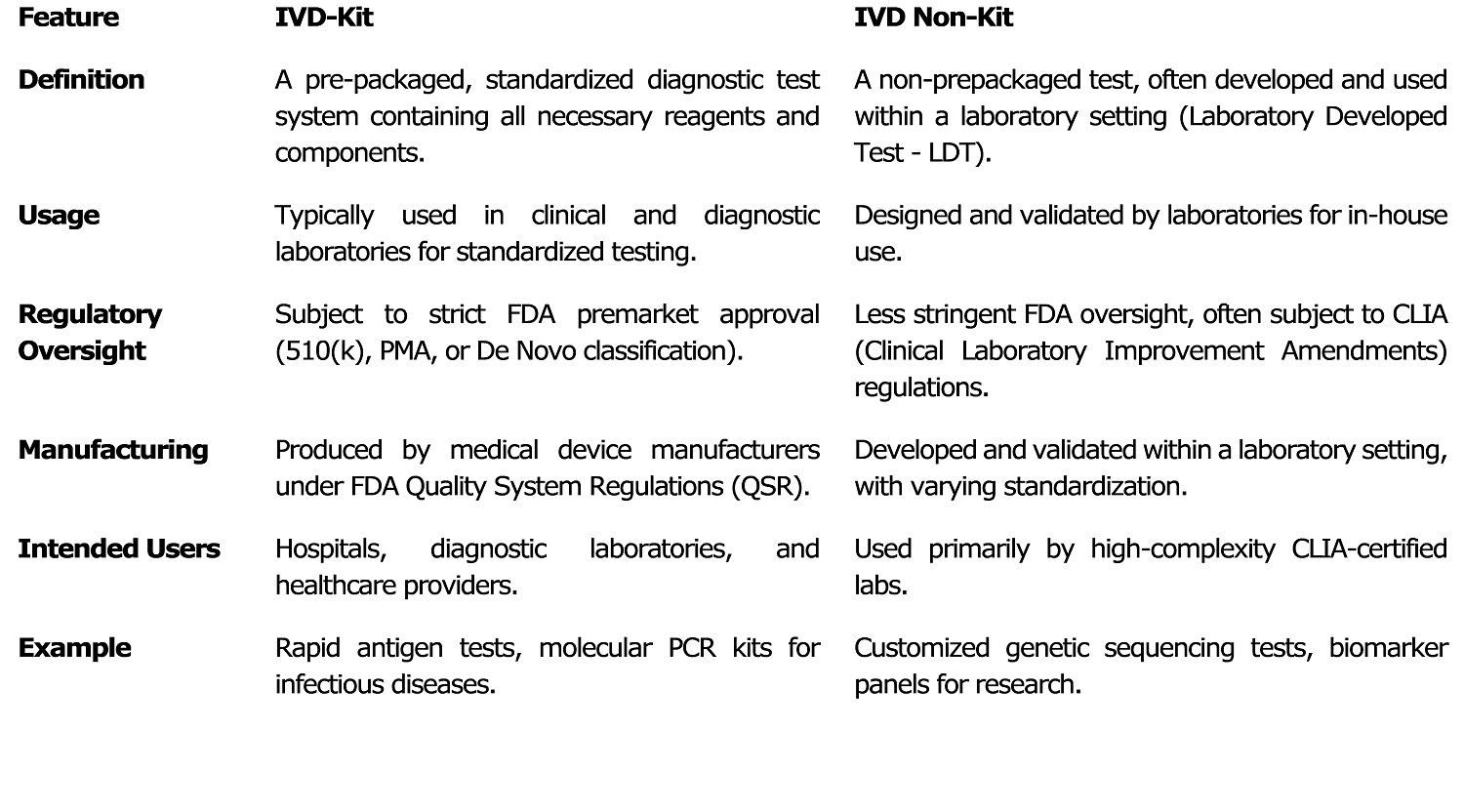
An IVD Kits is a complete diagnostic testing system that is pre-packaged with all necessary reagents and components, intended for use in hospitals, clinical laboratories, and diagnostic centers. These kits must comply with strict regulations established by the USFDA, which may involve obtaining 510k clearance, Pre-Market Approval (PMA), or De Novo submission.
They are manufactured in large volumes by medical device companies in accordance with FDA Quality System Regulations (QSR). The approval process for IVD Kits requires extensive premarket testing, including validation of both analytical and clinical performance. Examples of IVD Kits include COVID-19 RT-PCR Kits, HIV ELISA Kits, and Pregnancy Test Kits.
In contrast, an IVD Non-Kit is a non-prepackaged test, typically developed and validated within a laboratory setting, also referred to as a Laboratory Developed Test (LDT).
These are primarily used for internal testing within laboratories and are regulated under the Clinical Laboratory Improvement Amendments (CLIA), with some requiring FDA oversight, particularly for high-risk tests. Non IVD Kit do not require extensive FDA premarket approval unless deemed high-risk. Examples include genetic sequencing panels and biomarker tests for rare diseases.
Regulatory Process for IVD Kits
IVD Kits are subject to strict FDA approval before commercial distribution. The regulatory submission pathway depends on the device’s risk classification. The 510(k) Clearance is applicable to Class II devices that demonstrate substantial equivalence to an already FDA-approved device.
Pre-Market Approval (PMA) is required for Class III high-risk devices, necessitating extensive clinical data. De Novo Classification is an option for novel devices that pose low-to-moderate risk without an existing predicate. Emergency Use Authorization (EUA) is granted for temporary approval in response to public health emergencies, such as COVID-19 test kits.
IVD Kit must undergo rigorous testing to ensure performance accuracy and reliability. Analytical performance testing includes sensitivity, specificity, limit of detection, precision, reproducibility, stability, and interference studies. Clinical performance testing involves real patient sample validation, comparator studies with predicate devices, and diagnostic accuracy evaluation through multi-center clinical trials for high-risk devices.
Clearance requirements for IVD Kit components depend on their criticality. Each component of an IVD Kit (e.g., reagents, primers, probes, and controls) may require individual FDA clearance or approval if they are considered critical to test performance. While some components such as reagents, primers, probes, and controls require FDA clearance, others classified as General-Purpose Reagents (GPRs) may not. Manufacturers must demonstrate that all components function effectively as a system. Compliance with Good Manufacturing Practices (GMP) under FDA’s 21 CFR Part 820 is mandatory, along with risk management documentation per ISO 14971 and proper labeling requirements such as Unique Device Identification (UDI) and performance data.
Post-market surveillance for IV -Kits includes continuous monitoring of device performance, reporting of adverse events and recalls as per 21 CFR Part 803, and participation in proficiency testing programs.
Regulatory Process for Non IVD Kit (Laboratory Developed Tests – LDTs)
Non IVD Kit device, commonly referred to as Laboratory Developed Tests (LDTs), are designed, validated, and used within a single laboratory. They are not mass-produced and are primarily regulated under CLIA to ensure laboratory testing quality and accuracy. USFDA oversight is generally limited unless the test is high-risk, in which case it may require PMA or 510(k) clearance. Certain states impose additional regulatory requirements on LDTs.
Testing requirements for Non IVD Kits include internal validation studies to ensure test accuracy and reliability. Analytical performance validation involves precision, accuracy, reproducibility, sensitivity, specificity, reference range establishment, and cross-reactivity analysis. Clinical validation includes retrospective or prospective studies, evaluation against reference methods, and real-world patient sample testing.
Quality control for IVD Non Kit involves regular internal assessments, participation in external proficiency testing programs, and ongoing documentation of test performance. Unlike IVD Kit, post-market surveillance is generally limited. Laboratories are encouraged to monitor test performance through periodic revalidation, and while adverse event reporting is not mandatory unless USFDA-approved, compliance with HIPAA (Health Insurance Portability and Accountability Act) regulations for patient data confidentiality is required.
Conclusion
IVD Kits and IVD Non-Kits devices (LDTs) serve critical roles in diagnostic testing but follow distinct regulatory pathways. IVD Kits require rigorous US FDA approval, extensive validation, and compliance with manufacturing regulations, whereas Non IVD Kits primarily operate under CLIA with internal validation protocols. Understanding these differences is crucial for developers, manufacturers, and laboratory professionals to ensure regulatory compliance, quality assurance, and patient safety.
References
- S. Food and Drug Administration (FDA). “In Vitro Diagnostics.” https://www.fda.gov
- Centers for Medicare & Medicaid Services (CMS). “Clinical Laboratory Improvement Amendments (CLIA).” https://www.cms.gov
- ISO 14971: Medical Devices – Application of Risk Management to Medical Devices.
- Clinical and Laboratory Standards Institute (CLSI) Guidelines for IVD Validation.
- College of American Pathologists (CAP) Accreditation and Guidelines for Laboratory Testing.
- FDA 21 CFR Part 820 – Quality System Regulation.
- FDA Guidance for Industry and FDA Staff – Commercially Distributed In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only.
Author:
Ms. Suman Mishra (M. Pharm)
Regulatory Consultant, FDA Compliance | Medical Device
I3CGlobal