FDA Medical Device Reclassification — A Complete Regulatory Overview
US FDA Medical device reclassification is one of the formal regulatory processes of the U.S. Food and Drug Administration (FDA) that ensures medical devices are regulated under the appropriate level of oversight, based on current scientific knowledge, clinical experience, and risk assessment.
Reclassification applies to device types, not individual products, and is intended to align regulatory controls with real-world data on safety and effectiveness.
Medical Device Reclassification
It is the process by which the FDA changes the regulatory class of an existing device type when:
- New scientific evidence becomes available
- Post-market experience demonstrates a different risk profile
- Technological advancements reduce or increase safety concerns
- Existing regulatory controls are no longer appropriate
Reclassification ensures that regulatory burden is proportional to risk and that public health protections remain effective.
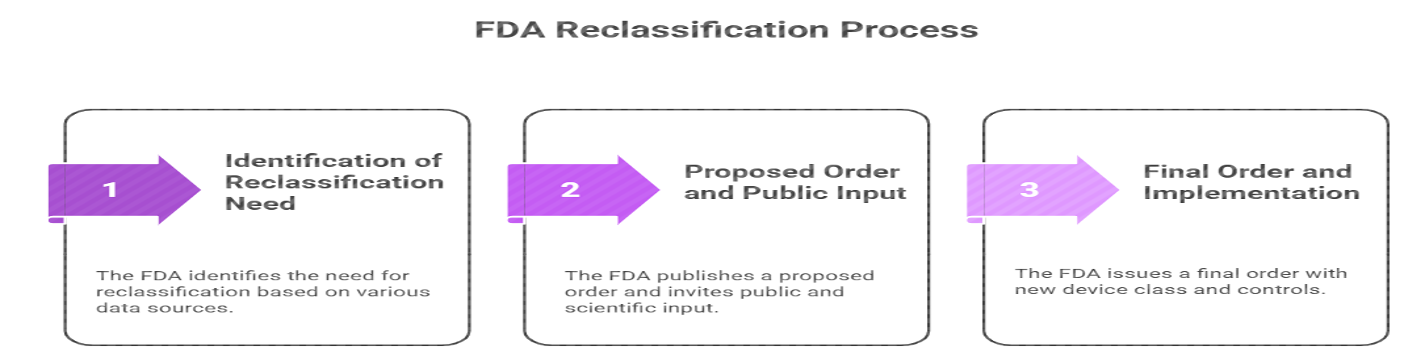
Legal Authority for Reclassification
The FDA’s authority to reclassify medical devices is established under the Federal Food, Drug, and Cosmetic (FD&C) Act.
- Section 513(e) — Reclassification of Classified Devices
- Section 513(f)(3) — Automatic Class III Devices
Regulatory relationship Between Reclassification and the De Novo Pathway
The De Novo classification pathway is closely linked to reclassification but applies to novel devices without a legally marketed predicate.
Key distinctions:
- Reclassification applies to existing device types
- De Novo applies to new, low-to-moderate risk devices
- A successful De Novo request establishes a new classification regulation
Once established, future devices of the same type may use the De Novo device as a predicate for 510(k) submissions.
Regulatory Impact on Manufacturers
Reclassification may significantly affect manufacturers by changing:
- Premarket pathway (PMA vs 510(k) vs exempt)
- Evidence requirements (clinical vs non-clinical)
- Applicable standards and special controls
- Labeling, performance testing, and post-market obligations
Manufacturers must reassess regulatory strategies following any final reclassification order.
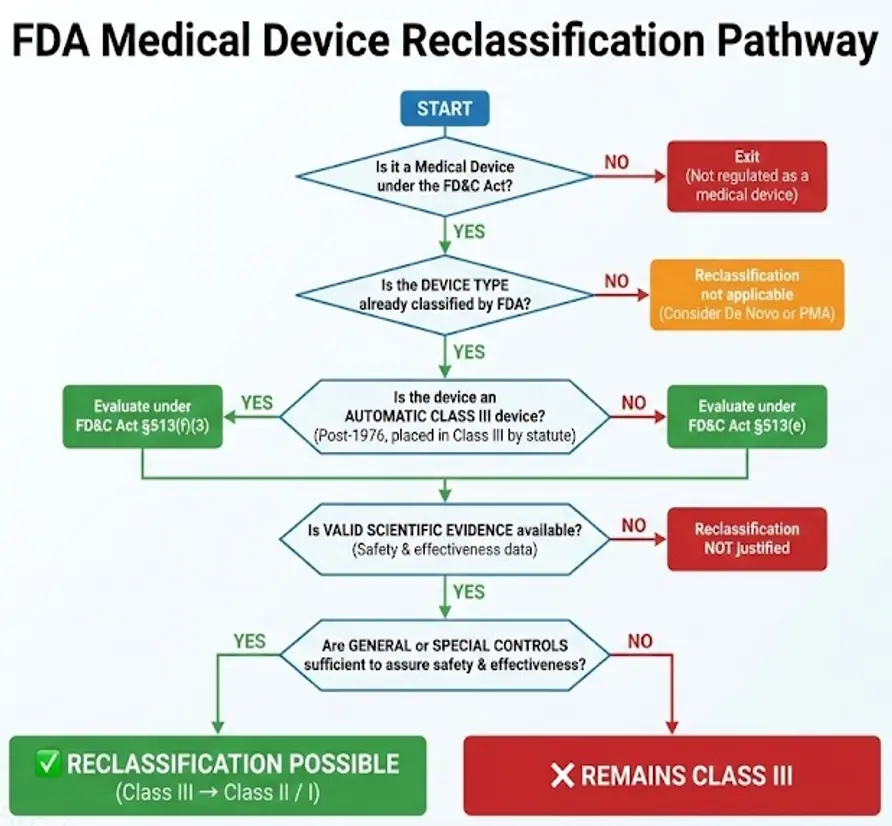
Conclusion
FDA medical device reclassification is a critical mechanism that maintains balance between innovation and patient safety. By continuously updating device classifications based on scientific evidence and real-world performance, the FDA ensures that medical devices are regulated with appropriate and effective controls.
Understanding reclassification is essential for manufacturers, regulatory professionals, and healthcare stakeholders navigating U.S. medical device compliance.