Quick Contact

Class IIa Medical Device
Class IIa medical devices constitute medium-risk devices such as orthodontic wires, surgical gloves, lancets, etc. For class 2a the declaration of conformity is backed up by notified body assessment post submission of technical documentation file. It holds a unique position within this classification system, embodying a delicate balance between innovation and patient safety.
Rold of Consultants in Class IIa CE Certification
It is a must to have experts on board who have previous experience with Class IIa medical device EU compliance and clinical evaluation for a successful outcome! Scope of I3CGLOBAL services are as follows:
- Class IIa Guidance
- Identification of standards
- Risk Analysis and Biological Evaluation
- Technical File Preparation
- Identifies test requirements and reviews the external reports
- Prepares Clinical Evaluation report as per Meddev 2.7/1 Rev 4.
- Prepares PSUR report
- Arrange Notified Body and coordinate with them till the issue of the CE Certificate
- Arrange EU Representative from the European Union
- EUDAMED Registration (SRN + Actor Registration)
- Arrange a Free Sale Certificate from the European Union
Do you need an email containing full details within 2 minutes?
Class IIa medical device CE Conformity Assessment Route
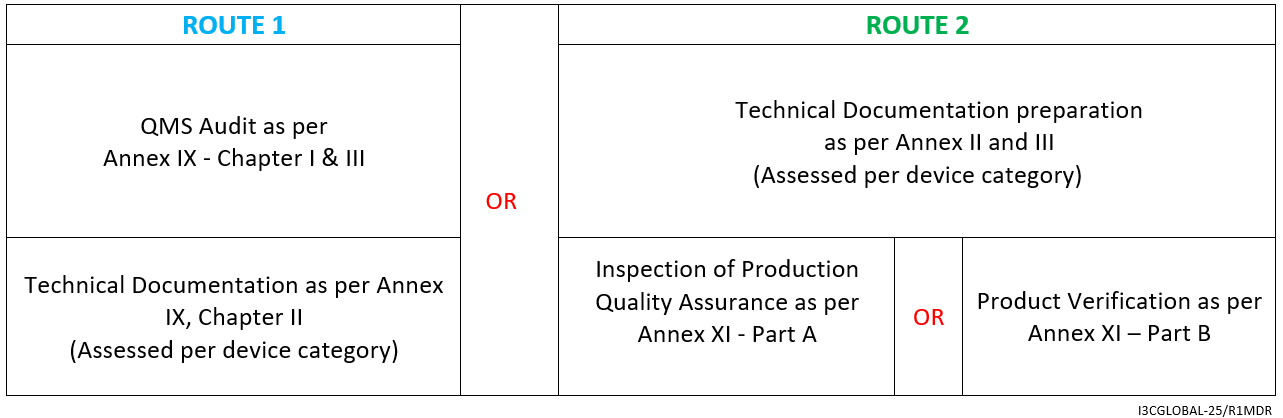
- Declaration of Conformity as per Annex IV
- Affixing the CE mark as per Annex V
Class IIa Medical Device Examples
Class IIa Medical Device encompass a wide range of medical technologies, often involving devices that come into contact with the body for diagnostic, therapeutic, or monitoring purposes. Some examples of Class 2a devices include:
1: Non-invasive Blood Pressure Monitors: Devices that measure a patient’s blood pressure without penetrating the skin fall under this category. These monitors are commonly used in clinics, hospitals, and home healthcare settings.
2: Infusion Pumps: Infusion pumps are used to deliver fluids, such as medications or nutrients, into a patient’s body in controlled amounts. They are vital in various medical treatments.
3: Contact Lenses: Non-corrective and corrective contact lenses for medical purposes fall under Class 2a. These lenses can have various applications, such as therapeutic or cosmetic use.
4: Diagnostic Ultrasound Devices: Ultrasound devices used for diagnostic imaging purposes, such as obstetric ultrasound, are classified as Class 2a. They are crucial tools in prenatal care and diagnostics.
5: Dental Filling Materials: Dental products like dental fillings and crowns that are intended to be placed within the oral cavity fall under this category.
Are you planning to obtain CE Certification through a Notified Body for class IIa Medical Devices or a System or a Kit? Contact us. We specialise in Documentation ensuring full compliance with all EU Regulatory requirements.
Frequently Asked Questions
Do I3CGLOBAL support with Notified Body slection
The CE Certification applicant/manufacturer is free to choose the NB as long as the notified body is accredited by the competent authority in the relevant EEA state, Switzerland, or Turkey and has the relevant product group within its scope
What is the preferred route for Class IIa CE Certification?
By chapters I and III of Annex IX OR compiling technical documentation by annexes II and III together with Conformity Assessment as specified in Section 10 or 18 of Annex XI
