Quick Contact

Class 1 Medical Device Importance
Class 1 medical device can be self-declared for CE marking compliance as per the MDR. Self-declaration means neither the notified body certification is required nor any other kind of approval from any certification bodies! Class 1 has the lowest perceived risk. In this case, the manufacturer can self-certify it.
Role of Consultants in Class 1 (s/m/r) Medical Device CE Marking
- Guidance and Technical File preparation
- Identifies test requirements and reviews the external reports
- Prepare Clinical Evaluation report as per Meddev 2.7/1 Rev 4.
- Arranges Notified Body and coordinates with them till the issue of CE Certificate
- Arranges European Authorized Representative from the European Union
- Arranges Free Sale Certificate from the European Union
Do you need an email containing full details within 2 minutes?
Steps for affixing Class 1
- Affixing the CE mark as per Annex V
- Technical File preparation as per Annex II and III
- Declaration of conformity preparation as per Annex IV
Sterile Class 1s Medical Device
Class 1s medical device have low/medium risks perceived. In this case of devices placed on the market in sterile condition, the Notified Body involvement is limited to the aspects of manufacture concerned with securing and maintaining sterile condition.
Measuring Class 1m Medical Device
Class 1m medical device have low/medium risks perceived. In this case of devices placed on the market with a measuring function, the Notified Body involvement is limited to the aspects of manufacture concerned with the conformity of devices with the metrological requirements.
Class 1r medical device
Class 1r medical device have low/medium risks perceived. In this case of reusable surgical instruments placed on the market, the Notified Body involvement is limited to the aspects relating to the re-use of the device in particular, cleaning, disinfection, sterilization, maintenance, and functional testing and related instructions for use.
CE Conformity Assessment Route
For Class 1s / 1m / 1r medical device (inspection is limited to sterility/metrology/re-use as applicable), the conformity assessment route is as follows:
Medical Device Technical File preparation as per Annex II and III
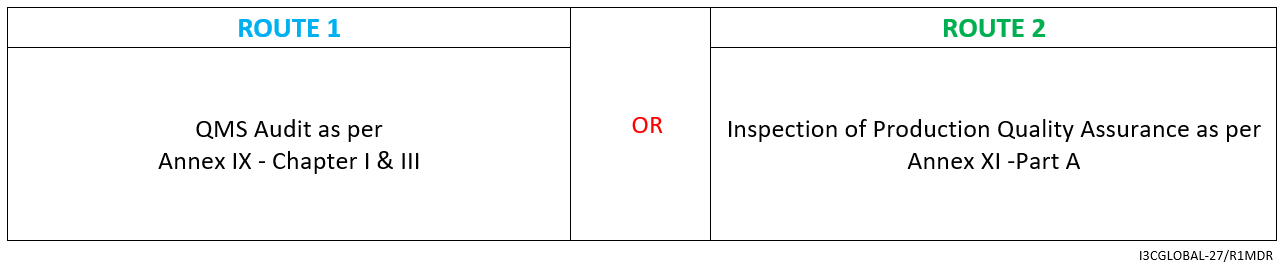
- Declaration of Conformity as per Annex IV
- Affixing the CE mark as per Annex V
Steps for placing Class 1 Medical Device on the EU market
- Confirm product as a medical device.
- Confirm product as a class 1s or 1m or 1r medical device.
- Confirm if the general safety and performance requirements have been met.
- Perform clinical evaluation.
- Prepare technical documentation.
- Request notified body involvement (For Class 1s, 1m & Ir)
- Prepare instruction for use and labeling.
- Check compliance with general obligations for manufacturers as established in Article 10
- The draw of the EU Declaration of Conformity
- Affix the CE marking.
Steps after placing Class 1 Medical Device on the EU market:
- Collect and evaluate Post Market Surveillance Data
- Vigilance system
- Non-conforming products -If a manufacturer finds that a device which they have placed on the market or put into service is not in conformity with the EU MDR, they will immediately take the necessary corrective action to bring that device into conformity, to withdraw it or to recall it, as appropriate.
How to build a Medical Device Technical Documentation as per MDR 2017/745? We have the answer and the right team to do it for you.


