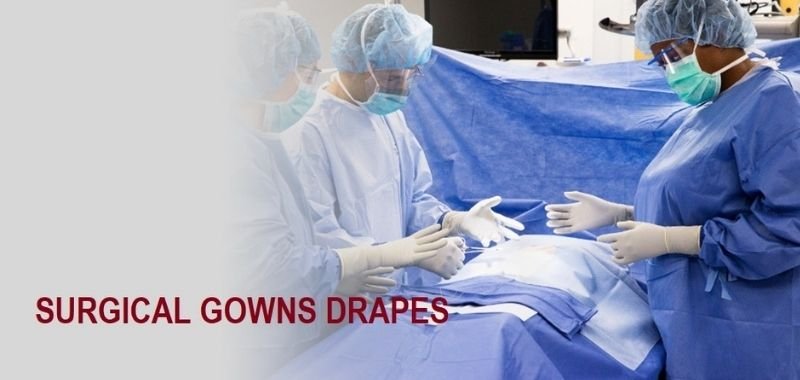
Surgical drapes are used to cover areas of the body other than those that need to be examined or operated on. During a medical examination or treatment, a paper or synthetic material covering is placed over the patient’s body.
Its purpose is to provide privacy or a sterile operating field. Surgical drapes are primarily used in sterile areas to separate and isolate the surgical site from other areas of the patient’s body. They are used to prevent surgical site infections by forming a barrier between the surgical field and potential infection sources. It aids in the reduction of contamination from non-sterile to sterile areas.
Types of surgical drapes and FDA regulation detailed below:
|
# |
Product | Product
code |
Regulation
Number |
Device Class
|
Submission |
| 1 |
Antimicrobial Drapes
|
PLY |
878.4370 |
2 | |
| 2 |
Cover, Barrier, Protective |
MMP |
878.4370 | 2 |
510(k) |
| 3 | Dental barriers and sleeves |
PEM |
878.4370 | 2 |
510(k) |
| 4 | Drape, Microscope, Ophthalmic |
HMW |
878.4370 | 2 |
510(k) exempt |
| 5 | Drape, Patient, Ophthalmic |
HMT |
878.4370 | 2 |
510(k) exempt |
| 6 |
Drape, Pure Latex Sheet, With Self-Retaining Finger Cot
|
EYX |
878.4370 | 2 |
510(k) exempt |
| 7 | Drape, Surgical |
KKX |
878.4370
|
2 |
510(k) |
| 8 | Drape, Surgical, Ent |
ERY |
878.4370
|
2 |
510(k) exempt |
| 9 | Drape, Surgical, Exempt |
PUI |
878.4370 | 2 |
510(k) exempt |
| 10 | Drape, Urological, Disposable |
EYY |
878.4370 | 2 |
510(k) exempt |
| 11 | Pad, Kelly |
FNW |
878.4370 | 2 |
510(k) exempt |
| 12 |
Ring (Wound Protector), Drape Retention, Internal
|
KGW |
878.4370 | 2 |
510(k) exempt |
| 13 | Microbial Sealant |
NZP |
878.4370 | 2 |
510(k) |
| 14 | Shave Prep Kit |
OJW |
878.4370 | 2 |
510(k) exempt Enforcement discretion |