Quick Contact
Class C IVDR Classification
IVDR Class C Classification refers to medium-to-high risk in vitro diagnostic devices, particularly those where incorrect results may lead to serious health consequences for patients or public health. Unlike Class A and B devices, Class C products require mandatory Notified Body involvement for both technical documentation and quality management system assessment. Manufacturers must demonstrate compliance with IVDR Annex I General Safety and Performance Requirements, supported by comprehensive analytical and clinical performance data.
Class C IVDs must also maintain a full technical documentation file, including risk management documentation, performance evaluation reports, post-market surveillance plans, and where applicable. These devices often include tests for infectious diseases, cancer markers, and blood grouping situations where false results could pose significant clinical impact. Class C IVDR typically demand rigorous verification and validation, including sensitivity/specificity studies, stability data, traceability, and robust labelling that ensures safe and effective use and thereby notified body approval.
From analytical validation to clinical performance, we build Class C IVDR dossiers that get cleared by Notified Bodies, not questioned. Contact us for more details or ask for detailed proposal.
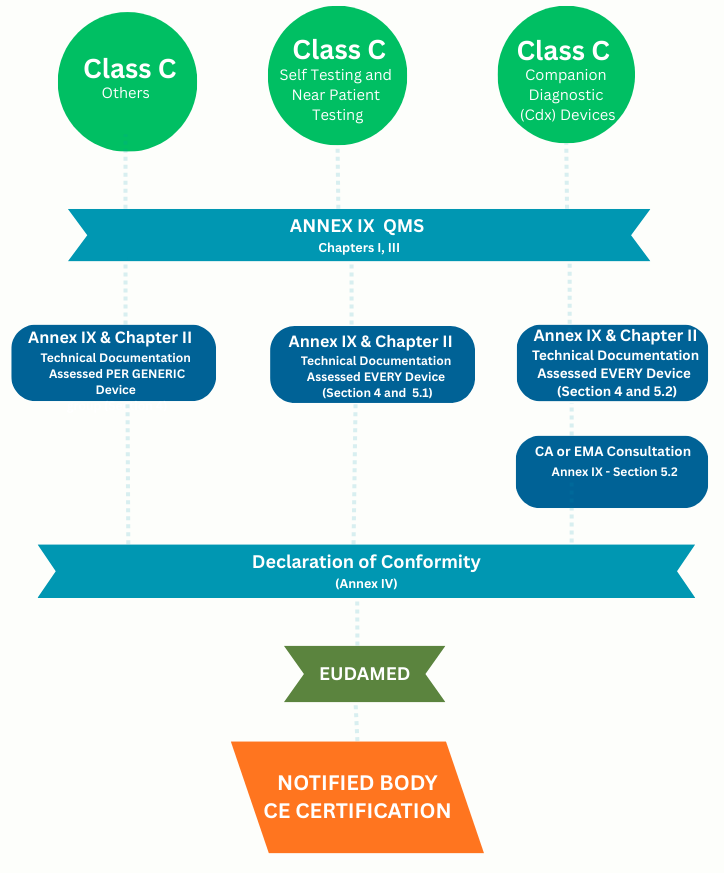
New IVDR Class C Conformity Assessment Routes
Transition Provisions for IVDD Devices Now Requiring NB Involvement Under IVDR Class C
If your device was already on the market under IVDD with a Declaration of Conformity signed before 26 May 2022, and no significant changes have been made to the device’s design or intended purpose, and under IVDR it is now classified as Class C (requiring NB involvement), then these transitional rules apply by following the steps below.
-
The organisation must upgrade their Quality Management System (QMS) to IVDR by May 26, 2025.
-
An application must be lodged with a Notified Body before May 26, 2026 for the devices signed under self-declaration.
-
A formal agreement with the Notified Body must be signed before September 26, 2026.
Manufactures meeting above criterial can continue selling the devices on the market or put into service until December 31, 2028.
Do you need an email containing full details about IVDR Classification within 2 minutes? Share your email below: Privacy Policy>>
Frequently Asked Questions
Technical Documentation Assessed Per Generic Device - Explain?
Annex IX & Chapter II, the technical documentation assessed Per Generic Device in IVDR means, each generic device group must have its own technical documentation and reviewed by the Notified Body separately. Even if devices share technology or intended use, performance data, risk management, and clinical evidence must be specific to each group.
Do we need to maintain technical file for class A devices?
What level of clinical and analytical performance data is expected for Class C?
Class C devices require robust performance evidence demonstrating safety, effectiveness, and intended clinical benefit. The IVDR applicant must provide the below supporting evidences to NB via technical documentation.
- Analytical Performance documents and reports covering accuracy, precision, sensitivity, specificity, limit of detection, linearity, interference studies, reproducibility, etc.
- Clinical Performance documents correlation with clinical outcome, comparison with gold standard test methods, clinical sensitivity/specificity.
- Scientific Validity documents and reports covering biological rationale and clinical relevance of the analyte/marker.
All above evidence must be sufficient to support intended use, claims, population, and specimen type, to Notified Body review.
Can literature and external data support clinical evidence, or is prospective study required?
What are the key gaps manufacturers usually face during IVDR TD preparation?
Common GAPS observed when I3CGLOBAL team conducts GAP analysis are the following in 80% of the cases.
⚠ Insufficient analytical/clinical performance data
⚠ Weak literature assessment or outdated PER
⚠ Incomplete GSPR checklist & risk-benefit justification
⚠ Lack of method comparison studies with reference tests
⚠ Missing usability/label validation and shelf-life evidence
⚠ Poor documentation structure not aligned with Annex II/III format
⚠ Limited PMS/PMPF planning and real-world data collection
IVD manufacturers often underestimate IVDR effort for Class C, which requires a strong evidence package, organised documentation, and early Notified Body engagement forecasting certification journey may take 18-22 months in some cases.