Quick Contact

Performance Evaluation Plan (PEP)
EU IVDR 2017/746, the Performance Evaluation Plan (PEP) is a must prepared document to demonstrates how, when, what methodologies and what was done to demonstrate that an IVD device performs as intended, safely and effectively, throughout its lifecycle. It is primarily explained in IVDR Annex XIII, Part A. The PEP is not a one time document it must be updated throughout the lifecycle.
The three pillars of the PEP consists of data generated for below areas:
⇒ Scientific Validity of the analyte/marker actually relate to the clinical condition?
⇒ Does the device measure the analyte correctly in terms of Precision, sensitivity, specificity, etc also know as Analytical Performance
⇒ Does the device yield results that lead to the correct clinical decision for the target patient or the Clinical Performance.
Why Performance Evaluation Plan (PEP)
A well developed plan helps to ensure the evaluation process is in line with guidlines and provides a documented which explains the scientific rationale for the methodologies established and followed by the manufacturer. It is imperative for the plan to incorporate acceptance criteria for the methodologies utilized, along with delineating the method by which the acceptability of the benefit-risk ratio will be assessed.
Practical steps for drafting and well formatted PER plan includes the following
- Every performance claim in your PEP must link back to your Intended Use Statement. If you claim “early detection,” your plan must include studies that prove performance in early-stage patients.
- For “legacy” devices (those already on the market), use the PEP to identify where your existing data fails to meet the stricter IVDR standards.
- Ensure you identify Certified Reference Materials or reference procedures to allow for metrological traceability of your results.
- If your IVD is software-based (SaMD), the PEP must add supporting evidence and detail the reference databases and data sources used for its decision-making.
Do you need an email containing full details within 2 minutes?
Core Sections of Performance Evaluation Plan as per Annex XIII (Part A)
| Section Title | Mandatory Content |
|---|---|
| General Performance Evaluation Requirements | Planning, conduct, and documentation of performance evaluation demonstrating compliance with Annex I GSPRs. |
| Device Identification | Device name, trade name, model(s), version, UDI (if applicable), and device classification. |
| Intended Purpose | Medical purpose, analyte(s), specimen type, testing principle, intended user, and use environment. |
| Target Population | Intended patient population, disease condition, demographics, and clinical setting. |
| Performance Claims | Clearly defined, measurable performance claims from IFU, labeling, and promotional materials. |
| Scientific Validity Claims | Claims demonstrating association between analyte and clinical condition. |
| Analytical Performance Claims | Accuracy, precision, sensitivity, specificity, LoD, LoQ, linearity, etc. |
| Clinical Performance Claims | Diagnostic sensitivity, specificity, predictive values. |
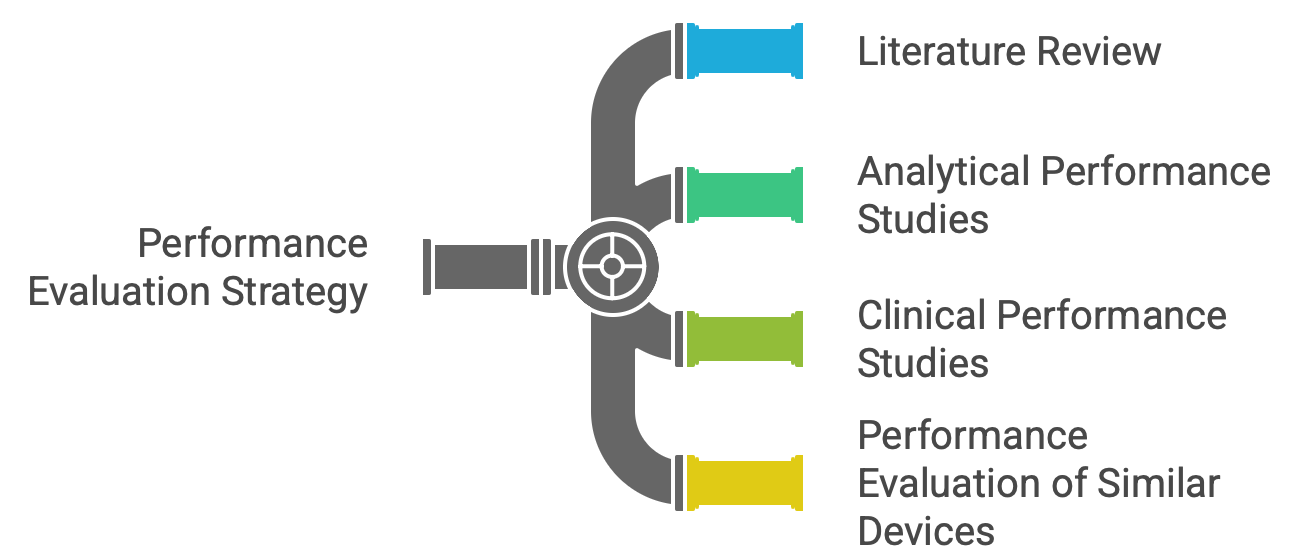
| Performance Evaluation Strategy | Strategy to demonstrate scientific validity, analytical, and clinical performance. |
| Performance Evaluation Data Planning | Identification, generation, and appraisal of performance evaluation data. |
| Acceptance Criteria | Pre-defined acceptance criteria with state-of-the-art justification. |
| Data Sources and Methodology | Literature search, study protocols, and data appraisal methods. |
| Lifecycle Performance Evaluation | PMS and PMPF integration, periodic review, and defined update triggers. |
Consultants Role in Performance Evaluation Plan (PEP)
To develop a Performance Evaluation Plan (PEP) for high risk Class C and D devices, I3CGLOBAL provides a simple methodology for data collection which is accepted by Notified Body and well for the final study ready strategy. For high risk devices, the PEP is the most critical document in the submitter Technical Documentation File because it dictates what clinical evidence manufacturer must collect.
Our experts helps define the Clinical Development Plan (CDP), which is a mandatory part of the PEP for Class C and D devices:
Gap Analysis: We assess your Legacy data (from IVDD) to see if it meets the rigorous IVDR Annex XIII requirements.
Study Design: For Class C and D, literature alone is not sufficient. We interact with CRO / CMO for design protocols for Prospective Clinical Performance Studies to ensure the data collected will satisfy a Notified Body.
Expert Panel Preparation: For Class D devices, the manufacturer is responsible for the documentation required for EU Expert Panel and EURL reviews, which may be prepared with the regulatory support of I3CGlobal
Drafting the Three Fundamental Sub-Plans: I3CGLOBAL experts author the technical components of the PEP, ensuring they are scientifically sound:
-
Scientific Validity Plan: Establish a systematic literature search protocol (per MDCG 2022-2) to prove the association between your analyte and the clinical condition.
-
Analytical Performance Plan: Define the specific sensitivity, specificity, LOD, LOQ parameters and acceptance criteria based on State of the Art (SOTA) standards.
-
Clinical Performance Plan: They outline how the diagnostic accuracy (PPV, NPV, likelihood ratios) will be validated in the target population.
Alignment with GSPR and Risk Management: A common reason for NB rejection is a lack of supporting evidence traceability. I3CGLOBAL ensures your PEP is integrated with GSPR (annex1) and Benefit risk.
PMPF and Lifecycle Maintenance: We draft the Post-Market Performance Follow-up (PMPF) Plan as part of the PEP to ensure continuous data collection after the device is on the market.
Coordination and Submission: If new clinical trials are needed, they coordinate with Clinical Research Organisations to ensure the study execution follows the PEP and act as your technical partner during the NB audit, responding to technical queries regarding the statistical methods or data choices made in the plan.
I3CGLOBAL and their professionals are qualified and experienced to takeup any risk class IVDR projects from any part of the world.
Frequently Asked Questions
Is a Performance Evaluation Plan (PEP) mandatory for all IVD devices?
Yes. A Performance Evaluation Plan (PEP) is mandatory for all IVD devices, irrespective of their risk class. The PEP defines the strategy, data sources, acceptance criteria, and methods used to generate and evaluate performance evidence. It must be established before market placement and maintained throughout the lifecycle, including post-market performance follow-up (PMPF).
What happens if gaps are identified during IVDR performance evaluation?
If gaps are identified between existing evidence and IVDR requirements, the manufacturer must perform a gap analysisand generate additional performance data as required. This may include analytical studies, clinical performance studies, or literature-based evidence. Unaddressed gaps are a common reason for Notified Body non-conformities or certification delays.
Very useful for small and medium size medical device manufactures

