Substantial Equivalence
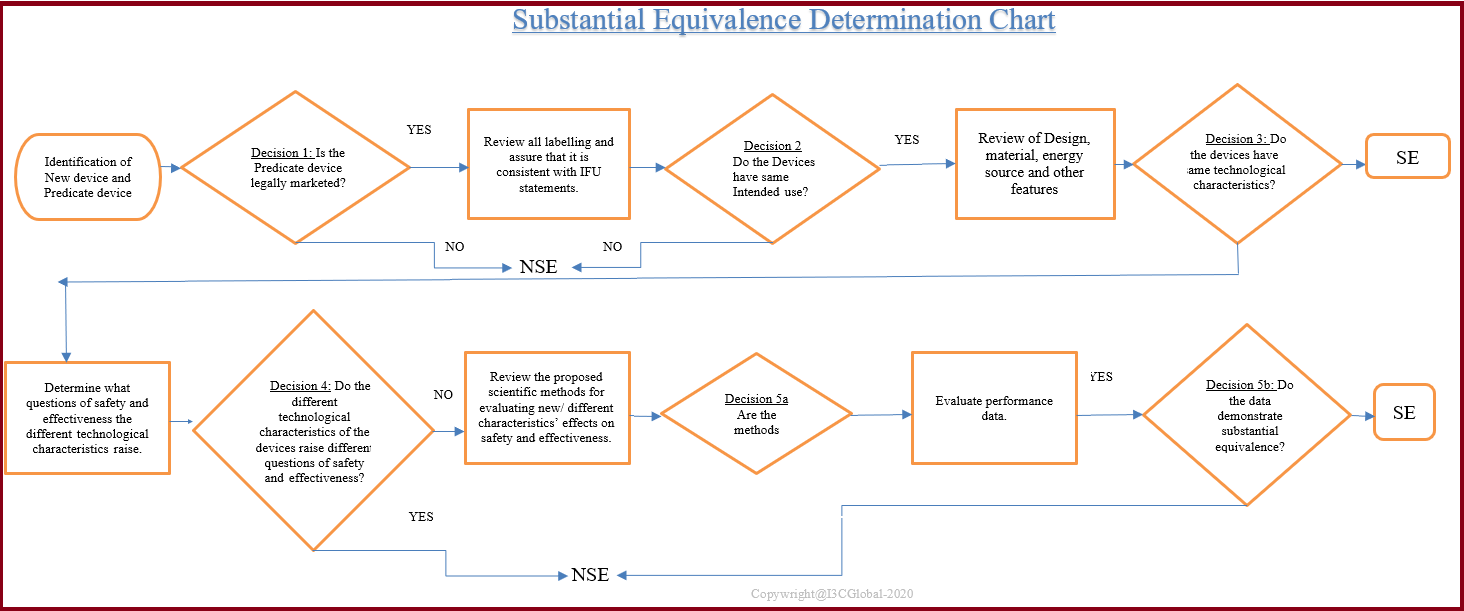
Substantial Equivalence is the demonstration that a new device, compared to a predicate device for the same intended use and the same technological characteristics and if differences in technological characteristics do not raise different questions regarding safety and effectiveness.
The crucial part of the evaluation is a selection of predicate devices. A legally marketed device previously cleared through the 510(k) process is considered a predicate device.
During the substantial equivalence determination, the intended use, the attributes in the labelling, and the technological characteristics and features of the device (dimensions, material, colour, etc.), clinical and non-clinical data were reviewed to establish whether the data demonstrate substantial equivalence.
A significant change in the materials, design or other features of the device does not raise different questions of safety and effectiveness and that the device is as safe and effective as a legally marketed device. Appropriate supporting data should be provided in concern to the safety and efficacy of the device.
Substantial equivalence doesn’t require the predicate device and the subjective device to be identical rather it can be demonstrated through intended use, design, materials, performance, safety, effectiveness, labelling, biocompatibility, and other applicable characteristics. Substantial equivalence can be claimed to a pre-amendment or post-amendment device that is legally marketed.
The pre-amendments device refers to devices which legally marketed in the U.S. before May 28, 1976. Devices for those regulation requiring a PMA application and has not been published by FDA and which have not been changed or modified significantly since then
If devices which are meeting the above criteria are called “grandfathered” devices and doesn’t require 510(k). The device should have the same intended use as of marketed product before May 28, 1976. If the device has a different intended use, then it falls under a new device and a 510(k) should be submitted to FDA for marketing clearance.
Post amendment devices refer to the medical devices which are marketed after May 28, 1976. In view of change in medical technology significantly since 1976, nearly all 510 (k) submissions claiming substantial equivalence to a post amendment device got cleared under the 510 (k) process recently.
Identifications of primary predicate device which is most similar to the device under review with respect to indications for use and technological characteristics shall be done by manufacturers. More than one predicate device may be identified by submitter to assist in demonstrating substantial equivalence in certain circumstances. Using split predicates are not consistent with the 510 (k) regulatory standard. In some cases Reference devices will be identified by manufacturers to support scientific methodology or standard reference values in their 510 (k) submission.