Quick Contact
UK MHRA Registration!
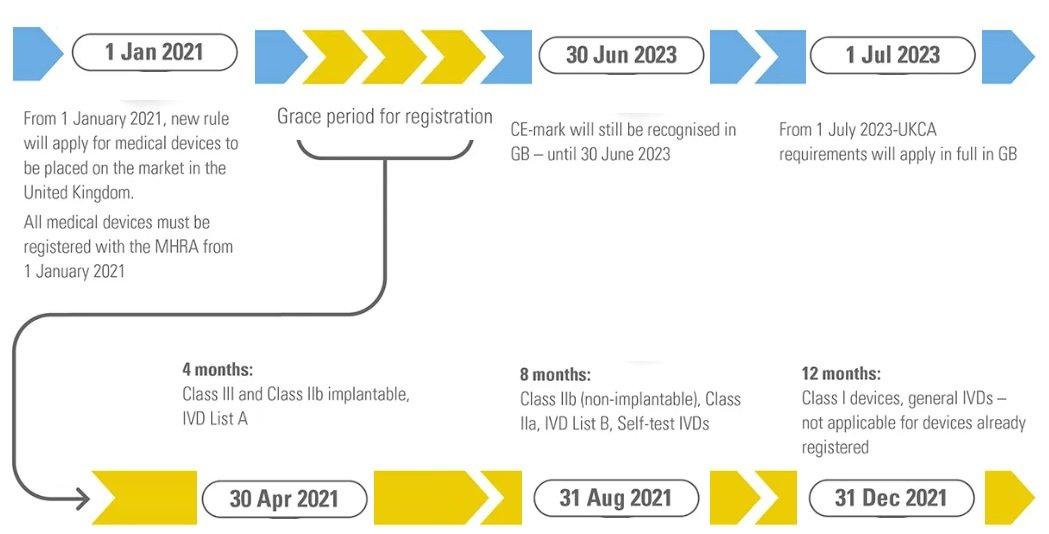
UK MHRA Registration for medical devices and in vitro diagnostics (IVDs) became mandatory on January 1st, 2021, for all products placed on the United Kingdom market. This registration requirement represents a critical regulatory milestone following Brexit, ensuring that all medical devices and In-vitro diagnostics sold in the UK meet stringent safety and quality standards.
The UK MHRA registration process is strategically staggered according to device risk classification and GMDN codes, allowing manufacturers adequate time to comply while maintaining market access. This phased approach balances regulatory oversight with practical implementation considerations.
UK MHRA Registration Renewal
UK MHRA Registration isn’t a one-time event. Manufacturers must renew their registration one year after initial application or confirmation, and subsequently every two years thereafter. This ongoing requirement ensures that device information remains current and accurate in the MHRA database.
Failure to renew your registration carries serious consequences: your records will be removed from the database, requiring a completely new registration application. More critically, you’ll lose the legal right to place your device on the UK market until registration is reestablished.
UK Responsible Person Requirements!
Mandatory for Non-UK Manufacturers: Under UK MDR 2002, all manufacturers based outside the United Kingdom must designate a UK Responsible Person (UKRP) before placing devices on the UK market.
This isn’t optional but it’s a legal prerequisite for market access. The UKRP must maintain a registered place of business within the UK and serves as the crucial link between foreign manufacturers and the MHRA. They facilitate registration processes, maintain documentation, and act as the point of contact for UK regulatory authorities.
Why You Need a UKRP: The UKRP requirement ensures that UK authorities have a domestic contact for regulatory matters, post-market surveillance, and compliance issues. Without a properly designated UKRP, foreign manufacturers cannot legally register with the MHRA or sell devices in the UK market, regardless of product quality or certification status.
Do you need an email containing full details about UK MHRA Registration within 2 minutes? Just share your email below:
UK MHRA Registration Timeline and Process
01
UKRP Agreement Preparation
Initial consultation and agreement drafting between manufacturer and UK Responsible Person, typically completed within 3-4 working days.
02
Documentation Assembly
Gather all required manufacturer information, device specifications, and technical documentation and GMDN Code.
03
MHRA Submission
Complete registration submission through MHRA systems, including all device listings and supporting documentation.
04
MHRA Review and Confirmation
MHRA processes registration application, typically requiring approximately 15 working days for standard submissions.
05
Registration Confirmation
Receipt of official UK MHRA Registration confirmation, enabling legal device placement on the UK market.
The entire process from initial UKRP engagement to final UK MHRA Registration confirmation typically spans 4-5 weeks under normal circumstances with I3CGlobal UK. However, timelines can vary based on documentation completeness, device complexity, and MHRA workload. Planning ahead and ensuring documentation accuracy are critical success factors.
Northern Ireland and EU Markets
(Special Considerations )
Northern Ireland Protocol
The regulatory landscape for Northern Ireland differs significantly from Great Britain due to the Northern Ireland Protocol. Manufacturers seeking to place devices in Northern Ireland must comply with EU regulations, not UK-specific requirements.
This means appointing a European Authorized Representative (EAR) rather than a UKRP, and obtaining CE Certification under EU MDR or EU IVDR regulations. Northern Ireland effectively remains aligned with EU medical device regulations, creating a dual-market scenario within the United Kingdom.
Dual Market Strategy
UK manufacturers aiming to serve both Great Britain and Northern Ireland/EU markets must navigate dual regulatory pathways. This requires maintaining both UKCA certification with a UKRP for GB, and CE marking with an EAR for Northern Ireland and the broader EU market.
While administratively complex, this dual approach ensures comprehensive UK and EU market access, maximizing commercial opportunities across all relevant territories.
UK MHRA Registration Search
Documents for UK MHRA Registration
Manufacturer and Facility Information
» Legal manufacturer name, address, designation, telephone and email
» Activity carried out by the legal manufacturer.
» Person responsible to communicate with MHRA (Name, Email & Contact Information)
» Mutually signed agreement with legal manufacturer and UK Responsible person.
Medical Device Information
» Basic UDI-DI ( Presently not enforced, but soon will come into effect)
» Medical device brand/trade or proprietary name
» Device model or versions
» IFU / User Manual / Catalogue reference number
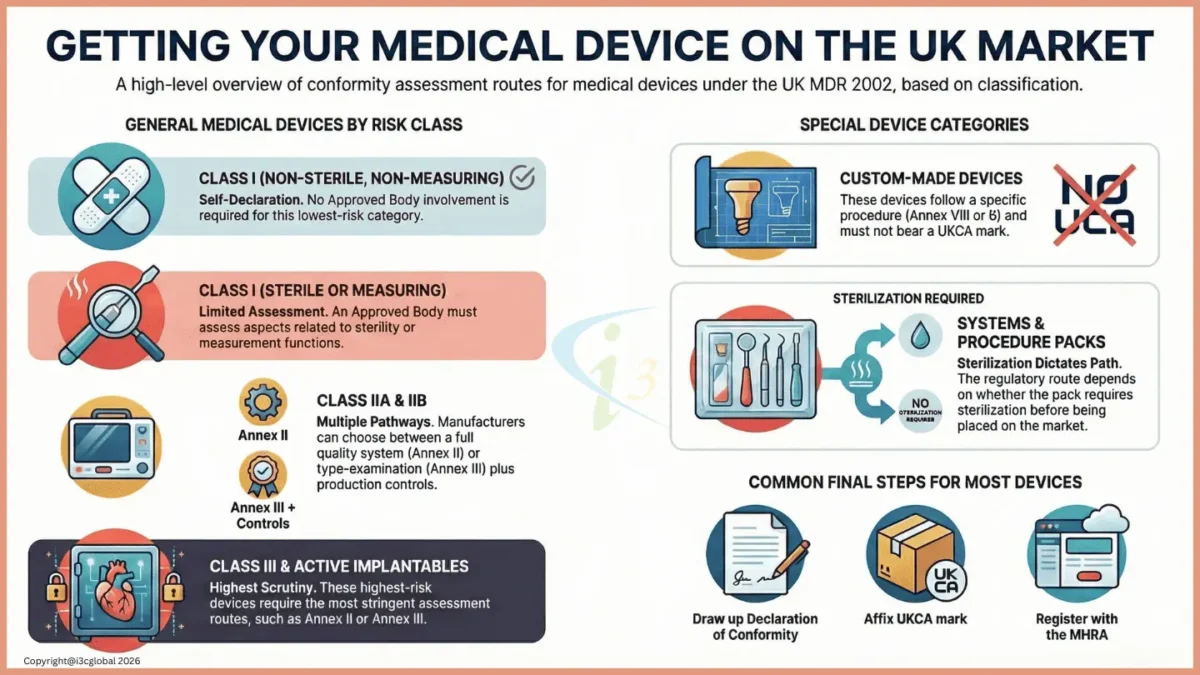
» UK Approved Body Or Notified Body name and address for all classes other than class I
» Type of Sterilization (If applicable)
» Details about Latex and phthalates.
» Medical Device MRI compatibility issues
» Conformity assessment certificates
» Signed and dated Declaration of Conformity
» Technical File latest revision number with date.
Partner with I3CGlobal for Your UK Market Access
I3CGlobal serves as the UK Responsible Person for foreign medical device manufacturers worldwide, providing comprehensive UK MHRA Registration and device listing services. Our expertise ensures smooth, compliant market entry while you focus on your core business.
Ready to Register? Get Started Today
Access our fee calculators, request detailed proposals, and learn more about our comprehensive UK Responsible Person and UK MHRA registration services. Our team is ready to guide you through every step of UK market access.
Frequently Asked Questions
Do I3CGlobal help with Technical Documentation?
Yes, we are medical device regulatory consultants and UKRP service providers. We help manufacturers submit technical documentation to Certification bodies for UKCA
Is I3CGlobal accredited with UK MHRA?
UKRP service providers are not accredited with UK MHRA. We are registered as a business establishment in the UK. We are providing service for many foreign service providers.
UKRP and UK MHRA Registration timeline
UKRP agreement preparation and mutual signing will be done in 3-4 working days and UK MHRA registration may take around 15 working days usually.
Placing goods in Northern Ireland
Manufacturers in the United Kingdom will need to designate a European Authorized Representative to place goods in the EU or Northern Ireland. CE Certification will be needed in Northern Ireland, and EU MDR and EU IVDR are applicable.
Devices with a CE marking can still be sold in the UK?
Devices with a CE marking can still be sold in the UK until June 30, 2023, but from July 1, all medical devices and in vitro diagnostics will require a UKCA certification to be sold in the United Kingdom.