Quick Contact

Class IIb Medical Device
Class IIb medical device, according to the new Medical Device Regulation (MDR 2017/745), are devices that are considered to pose medium to high risk. These devices include incubators for babies, intraocular lenses, orthopedic nails, plates, etc.
CE Certification of Class 2b medical device can be achieved by submitting the declaration of conformity with the technical documentation backed up by notified body assessment, and review of clinical evaluation consultation procedure by NB depending on the type of device.
Do you need an email containing full details within 2 minutes?
Class 2b Conformity Assessment Route
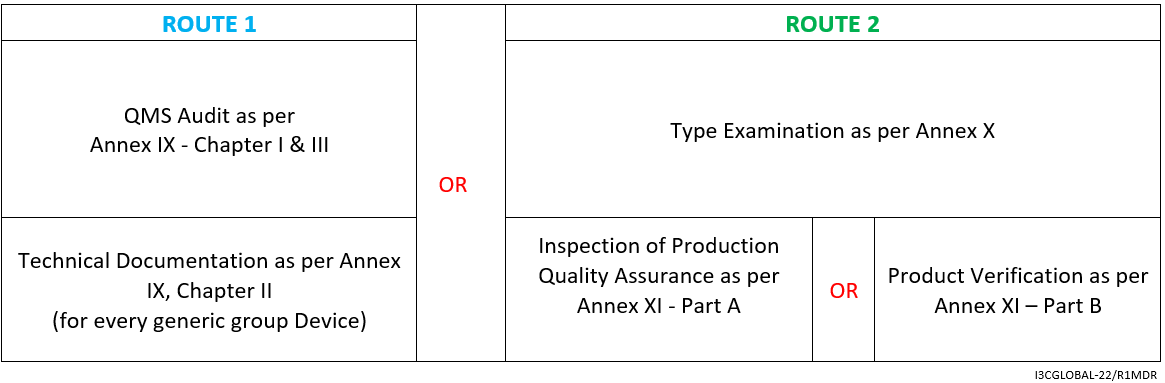
Followed by the above-given assessment methods, Class 2b Medical Devices falling under rule 12 must run through:
- Clinical Evaluation Consultation Procedure as per Annex IX sec. 5/Annex X sec. 6
- Declaration of Conformity as per Annex IV
- Affixing the CE marking as per Annex V CE marking
Excluding Rule 12 CE Conformity Assessment Route
Class IIb Implantable WET* Medical Devices, and Non-implantable (non-rule 12, non-WET) CE marking for medical devices Conformity Assessment Route
*Well-Established Technologies (WET) – sutures, staples, dental fillings and braces, tooth crowns, screws, wedges, plates, wires, pins, clips & connectors as per Article 52 of MDR.
The CE mark conformity assessment route is as follows:
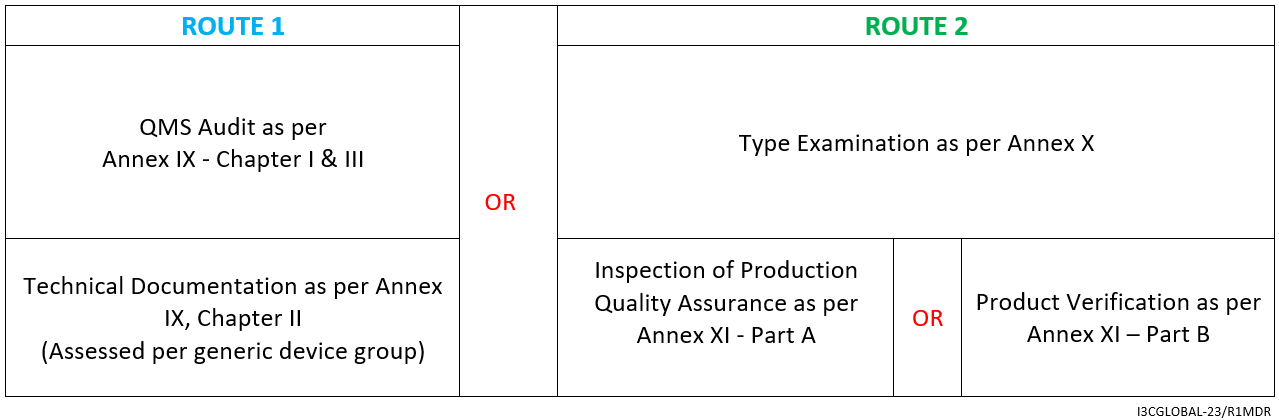
- Declaration of Conformity as per Annex IV
- Affixing the CE mark as per Annex V
Implantable Device CE Conformity Assessment Route
For Implantable Class 2b medical device (Excluding WET), the CE mark conformity assessment route is as follows:
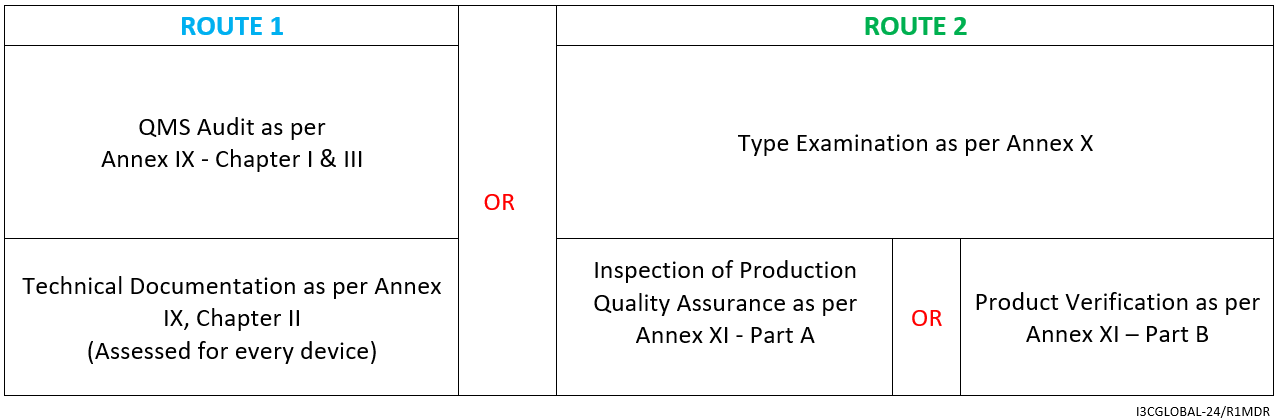
- Declaration of Conformity as per Annex IV
- Affixing the CE mark as per Annex V
Class IIb Medical Device Consultants
- Guidance and Technical File preparation
- Identifies Biocompatibility Test requirements and reviews the external Protocols and Reports.
- Prepares Clinical Evaluation Report as per Meddev 2.7/1 Rev 4
- Conducts Risk Analysis and prepares Risk Management File as per EN ISO 14971
- Arrange Notified Body and coordinate with them till the issue of the CE Certificate
- Arrange a European Authorized Representative from the EU
- Prepares Periodic Safety Update Report (PSUR), PMS plan and PMCF plan and report
- EUDAMED Registration
- Arrange a Free Sale Certificate from the European Union
Are you planning to obtain CE Certification for 2b Medical Device? We specialize in Technical Documentation by MDR 2017, ensuring full compliance with all Notified Body requirements.
Frequently Asked Questions
General route for Class IIb CE Marking
By Annex IX (including a technical documentation review of at least one representative device per generic device group) however, for implantable Class 2b medical device (exceptions listed in Article 52) a technical documentation review is required for every device.
OR
Annex X (EU Type Examination) in combination with Annex XI