IVD Performance Evaluation Data and Relevance of the EU Population
In the context of the EU IVDR (Regulation (EU) 2017/746), the phrase “performance evaluation data and relevance of the EU population” has a very specific regulatory meaning. It is about proving that an in vitro diagnostic (IVD) device works as intended for patients and users in the European Union.
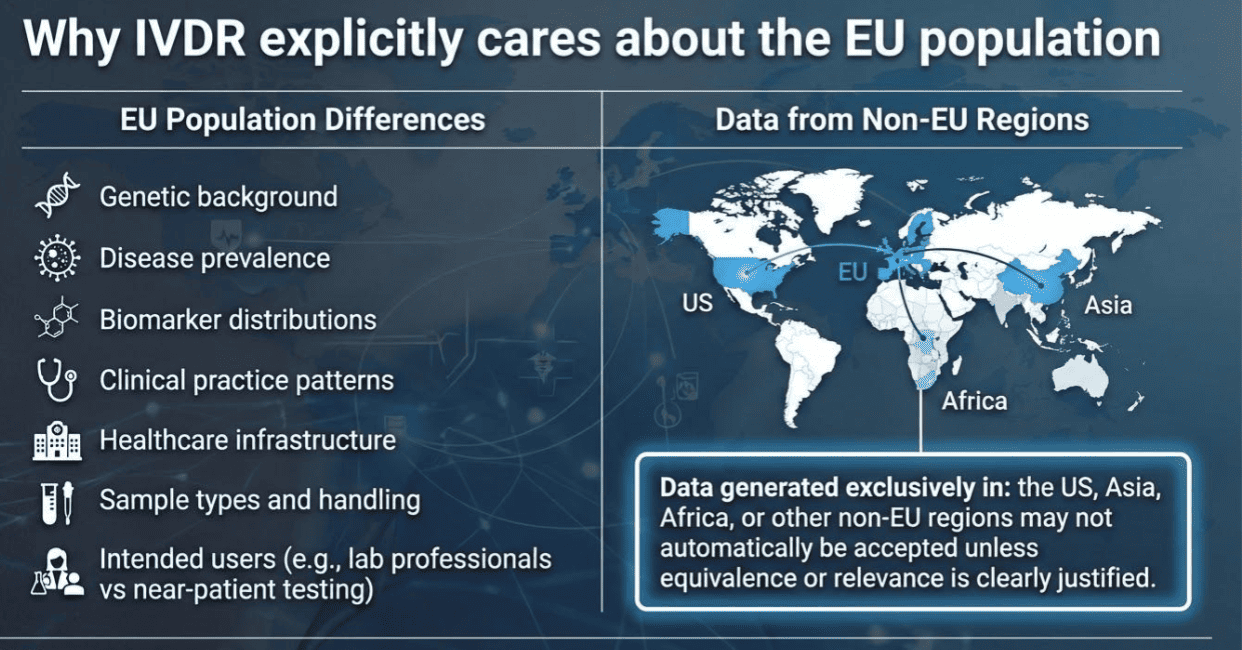
Relevance of the EU population” means that the performance evaluation data used to demonstrate conformity under the IVDR must be applicable to the population in which the device will be placed on the market and used, namely patients, donors, and users within the European Union. In practical terms, regulators are asking whether the data reliably demonstrates that the device will perform as intended for the EU population, taking into account factors such as demographic characteristics, disease prevalence, genetic or biological variability, clinical practice patterns, and healthcare settings in Europe. Where performance data are generated outside the EU, the manufacturer must justify that the study population and conditions are sufficiently comparable to those in the EU so that the results can be scientifically extrapolated and remain valid for EU use.
IVD Performance Evaluation Data and Relevance of the EU Population
Manufacturers must justify that their performance data is relevant to the EU population by showing one or more of the following:
A. EU-based data (strongest case)
-
Clinical or analytical studies conducted in EU sites
-
EU patient samples
-
EU laboratories and users
B. Non-EU data with justification (acceptable, but must be defended)
If data is from outside the EU, the manufacturer must explain:
-
Why the population is comparable to the EU population
-
That ethnicity, disease prevalence, and clinical context do not affect performance
-
That EU standards of care are reflected
Example justification:
“The study population is comparable to the EU population in terms of age, sex distribution, disease prevalence, and biomarker expression.”
Where this requirement appears in IVDR
Key references are mentioned in the following section of IVDR
-
Article 56 – Performance evaluation and demonstration of conformity
-
Annex I (GSPR) – General Safety and Performance Requirements
-
Annex XIII – Performance Evaluation and Performance Study requirements
-
Annex XIV – Post-Market Performance Follow-up
Notified Bodies routinely ask:
-
“Is the study population representative of the EU population?”
-
“Why are there no EU sites?”
-
“Provide justification for extrapolating non-EU data to the EU population.”
Legacy IVDD devices, demonstrating relevance to the EU population is no longer implicit or assumed; it must be explicitly documented, scientifically justified, and continuously supported throughout the device lifecycle in line with IVDR Articles 56 and Annex XIII and XIV