FDA Medical Device Classification
FDA Medical Device Classification consists of three distinct classes based on the level of risk they pose to patients and users. Each device class requires a different level of regulatory controls to ensure the safety and effectiveness of the device.
Understanding these classifications is important for manufacturers / Initial exporters / initial importers/specification developers seeking FDA clearance. The FDA medical device classification is different from the European classification.
For reliable, cost-effective, and fast services related to US FDA Medical Device Classification and device compliance, get in touch with us.
FDA Medical Device Classification and Specialties Covered
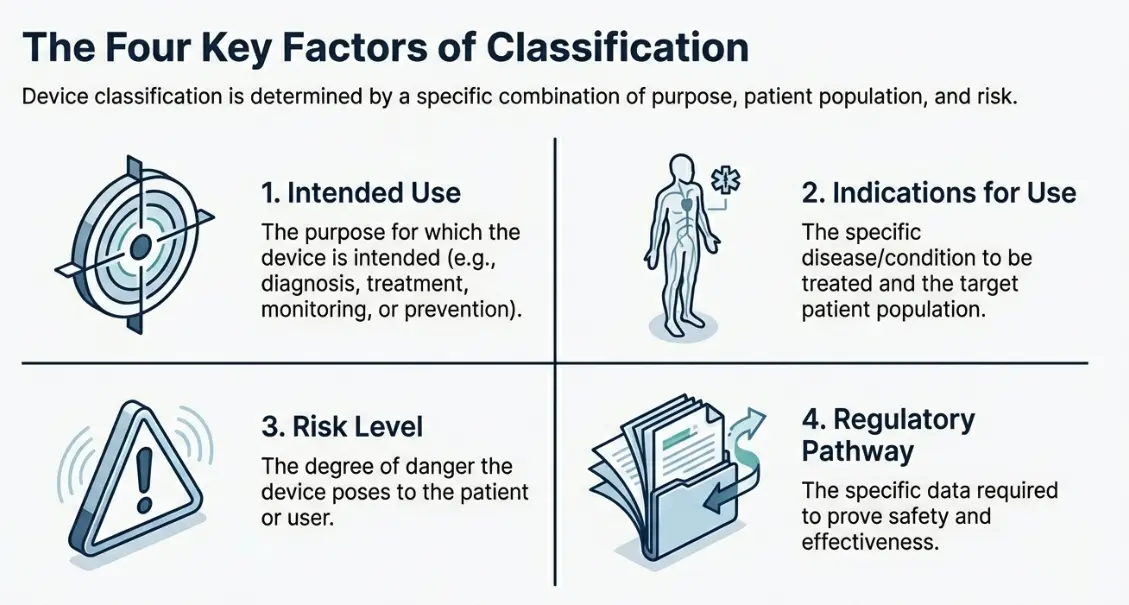
Key Factors of US FDA Medical Device Classification!
| Parameter | Description |
|---|---|
| Intended Use | The purpose for which the medical device is intended, including whether it is used for diagnosis, treatment, monitoring, prevention, or mitigation of a disease or condition. |
| Indications for Use | A description of the specific disease(s) or condition(s) the device is intended to diagnose, treat, prevent, cure, or mitigate, including details of the target patient population. |
| Risk Level | The level of potential risk posed by the device to the patient, user, or third party, considering factors such as invasiveness, duration of use, and potential for harm. |
| Regulatory Pathway | The applicable regulatory classification and approval route, based on the level of clinical, performance, and safety data required to demonstrate compliance with applicable regulations and standards. |
FDA Medical Device Classification System: Requirements for Class I, II & III
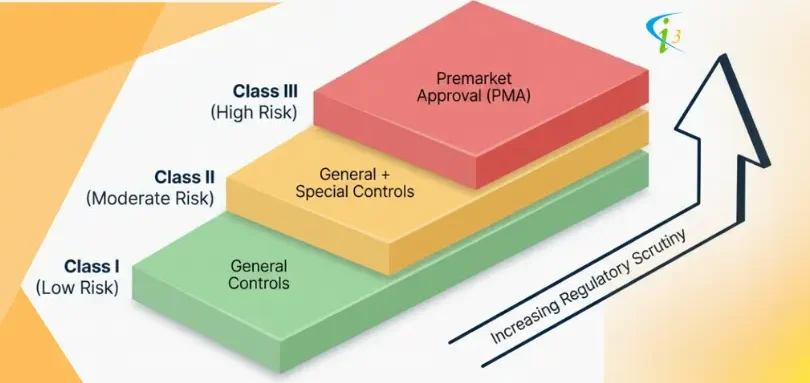
The US FDA has classified medical devices into three classes. They are Class I, Class II, and Class III, and each corresponds to increasing levels of risk
Class I - Low Risk (General Controls)
Class I medical devices are considered to pose the lowest risk to patients and users. Most of the class 1 devices are exempt from the 510(k) clearance process, though some may still require 510(k) clearance for selling in the USA.
Examples of Class 1 devices that are exempt from 510(k) include bandages, tongue depressors, manual stethoscopes, and non-powered wheelchairs. However, certain devices like examination gloves still require 510(k) clearance despite being classified as Class 1.
The regulatory controls established by the FDA for class 1 devices are the following:
- Establishment Registration and Device Listing
- US Agent for Foreign manufactures
- Labelling
- Reporting of adverse events or malfunctions
- Compliance with FDA regulations on GMP (21 CFR 820) superseded with QMSR now
Most class I devices are not required to submit a 510(k), making them easier to sell in the USA market.
Class II - Moderate Risk (General & Special Controls)
Class II medical devices pose a higher risk than class I devices and require special controls in addition to general controls. Most Class II devices require 510(k) clearance which involves demonstrating that the device is substantially equivalent to an already legally marketed device (known as a predicate device).
Examples of Class II devices are Powered wheelchairs, surgical drapes, infusion pumps, and diagnostic imaging devices.
The regulatory controls (Special Controls) established by the FDA for class II devices are the following
- Performance standards
- Post-market surveillance
- Special labelling requirements
- Clinical performance data
- Establishment Registration and Device Listing
- US Agent for Foreign manufactures
- Reporting of adverse events or malfunctions
- Compliance with FDA regulations on GMP (21 CFR 820) superseded with QMSR now
A 510(k) clearance is required for Class II devices unless the device is specifically exempt. This submission demonstrates that the new device is equivalent to a predicate device already on the market.
Class III - High Risk (General Controls & PMA)
Class III medical devices possess the highest risk are mainly used to sustain or support life, and are implanted. These devices require Premarket Approval (PMA) from the FDA to demonstrate safety and effectiveness.
Examples of Class III devices are Implantable pacemakers, heart valves, and breast implants.
The regulatory controls follow Premarket Approval (PMA), requiring clinical trial data to ensure the device’s safety and effectiveness. The FDA closely scrutinizes these devices because they are crucial in life-supporting or high-risk medical scenarios.
FDA Medical Device Classification & Regulatory Pathways for Device Approvals
| FDA Regulatory Pathway | Description | Applicable Device Class |
|---|---|---|
| FDA 510(k) Premarket Notification | This is the most common route for market entry. A manufacturer or specification developer must demonstrate that the device is substantially equivalent to a legally marketed predicate device. In most cases, extensive clinical trials are not required. |
Class I (most exempt) Class II |
| Premarket Approval (PMA) | Required for high-risk medical devices. This process involves clinical testing and a full scientific and regulatory review by the FDA to demonstrate that the device is safe and effective for its intended use. | Class III |
| De Novo Classification Process | Used for novel devices that do not have a legally marketed predicate device. If the device is determined to be low to moderate risk, the FDA may grant De Novo classification, assigning the device to Class I or II and avoiding the need for a PMA. | Class I or Class II |
Note:
- If your product is found to be “exempt,” just general regulations apply, and no formal FDA submission is necessary. You must, however, register your business with the FDA and then list the product.
- If you discover that your product requires specific controls, you will need to prepare a 510(k) filing to the FDA and obtain clearance before coming to market. Following that, you must register your business and list the product.
- If your product requires premarket approval, you must follow the FDA PMA process to obtain permission before going to market.