IVDR Classification
IVDR classification is based on the intended purpose and inherent risks, therefore they are classified A, B, C and D considering their intended purpose and inherent risks. Manufacturers must classify their in vitro diagnostic devices by the rules outlined in Annex VIII of Regulation EU 2017/746, commonly referred to as IVDR classification.
How IVDR are classified?
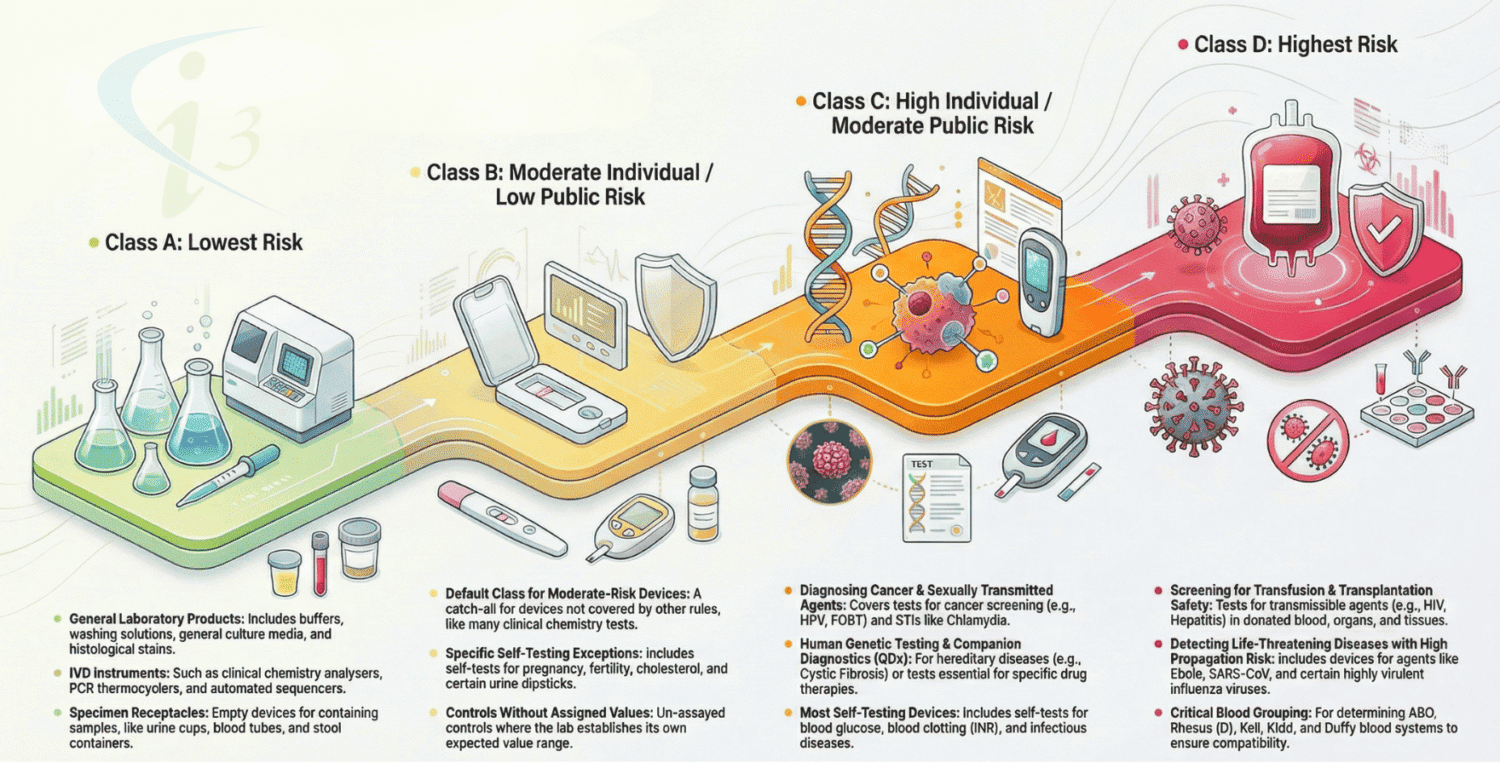
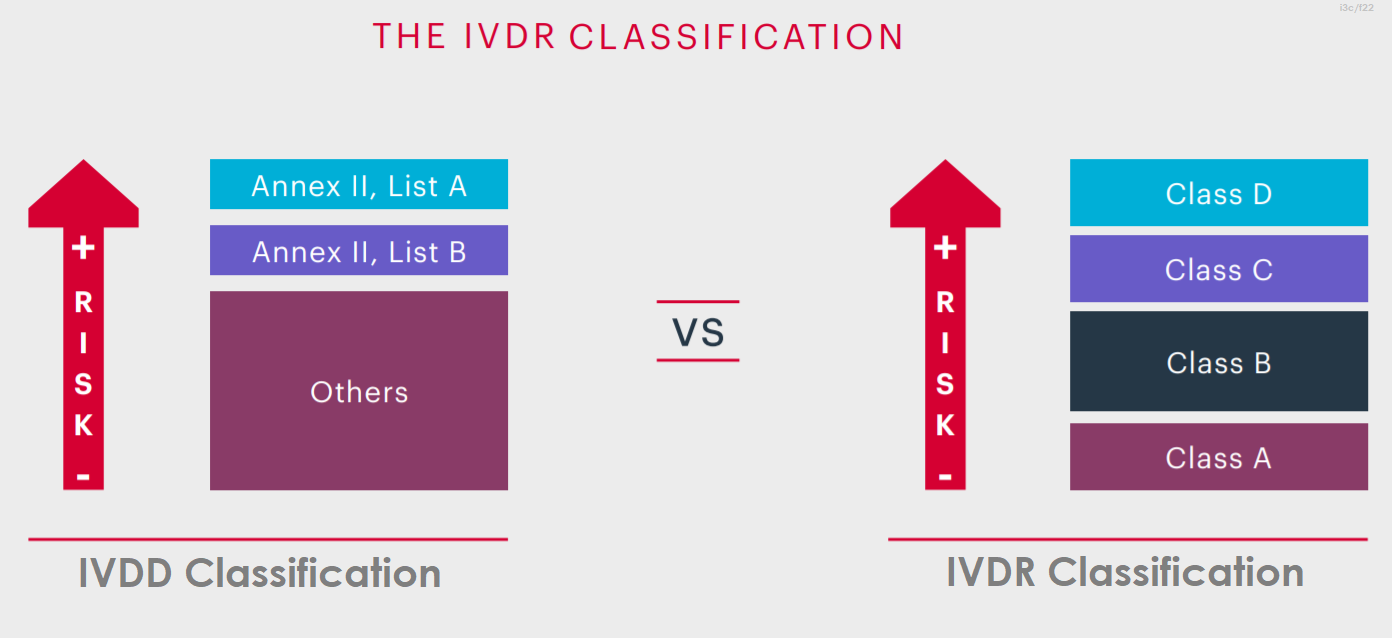
IVDR classified as A, B, C and D considering their intended purpose and their inherent risks. The lowest risk category at Class A, up to the highest at Class D. The new system for classifying IVD medical devices represents a significant departure from the current IVD directive, constituting one of the few radical changes introduced by the new regulations.
It represents an enhancement over the existing directive, aligning the classification of IVD medical devices with that of other devices and adhering to international practices advocated by the GHTF. Moreover, it offers a more comprehensive approach compared to the current directive, facilitating a smoother application to new IVD devices.
Do you need an email containing full details about IVDR Classification within 2 minutes? Share your email below: Privacy Policy>>
IVDR Classification Rules
The responsibility for identifying the applicable risk class of its IVD device lies with the manufacturer. However, for Classes B, C, and D devices, the notified body will verify the accuracy of this classification. Additionally, competent authorities may also verify the classification, including for Class A devices. Therefore, manufacturers must maintain a documented rationale for their classification decisions in the IVDR Technical documentation.
Examine all IVDR classification rules below thoroughly and ascertain which rule corresponds to the highest risk class applicable to the device. It’s conceivable that multiple rules may be relevant or that the device serves various intended uses. In such instances, the rule leading to the highest risk class must be applied.
Rule 1
- This Rule applicable for most of the devices in Class D such as devices intended to be used for the detection of the presence of, or exposure to, a transmissible agent in blood, blood components, cells, tissues or organs, or in any of their derivatives, in order assess their suitability for transfusion, transplantation or cell administration.
- Devices intended to be used for the detection of the presence of, or exposure to, a transmissible agent that causes a life threatening disease with a high or suspected high risk of propagation.
- Devices intended to be used for determining the infectious load of a life-threatening disease where monitoring is critical in the process of patient management.
Rule 2
- This rule applicable for most of the devices majorly in Class C and few in Class D
- Devices intended to be used for blood grouping, or tissue typing to ensure the immunological compatibility of blood, blood components, cells, tissue or organs that are intended for transfusion or transplantation or cell administration.
Rule 3
Generally majority of the IVD devices falls under this category are in Class C
- Device used for (a) sexually transmitted disease (b) foetus or embryo (c) pre-natal screening of women (d) infective disease status or immune status (e) screening, diagnosis, or staging of cancer (f) human genetic testing (g) screening for congenital disorders in the embryo or foetus (h) congenital disorders in new-born babies etc.
Rule 4
Majority of the Devices falls under Class C and few in Class B Intended for self-testing
- Note : exemption from devices for the detection of pregnancy, for fertility testing, Cholesterol / Glucose / Erythrocytes / Leucocytes / virus/ bacteria/ urine level determination.
Rule 5
Generally Class A devices falls under this rule.
General laboratory use accessories such as buffer solutions, washing solutions, culture media, histological stains used in IVD procedures and also some instruments for In Vitro procedures and specimen receptacles.
Rule 6
Class B Devices are not covered in any above IVDR classification (1-5) rules
Rule 7
Generally considered in Class B Devices
Devices which are controls without a quantitative or qualitative assigned value.