IVDR Technical Documentation Sampling
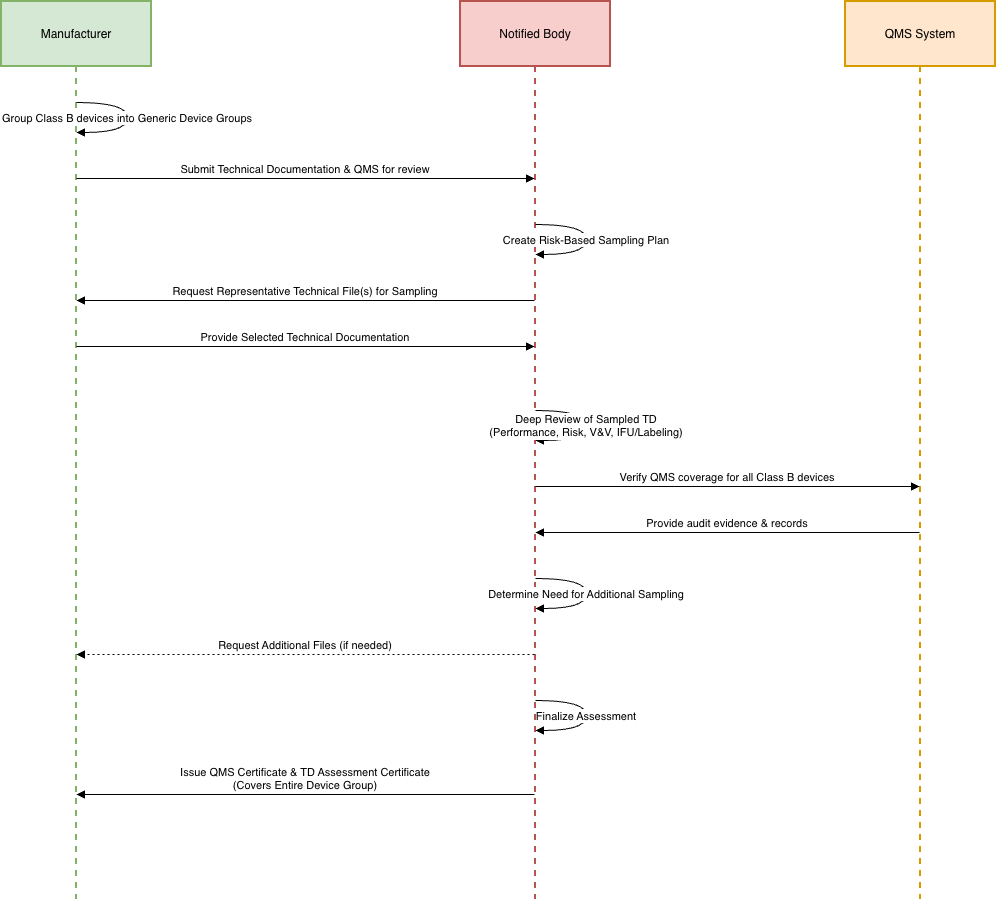
Typical Process for Technical Documentation (TD) Sampling for Assessment
Under EU IVDR 2017/746, Notified Bodies apply a structured sampling process to review Technical Documentation for devices covered under a manufacturer’s quality management system certificate. The goal is to ensure consistent compliance, product safety, and performance across the manufacturer’s full device portfolio. A typical sampling process includes the following steps:
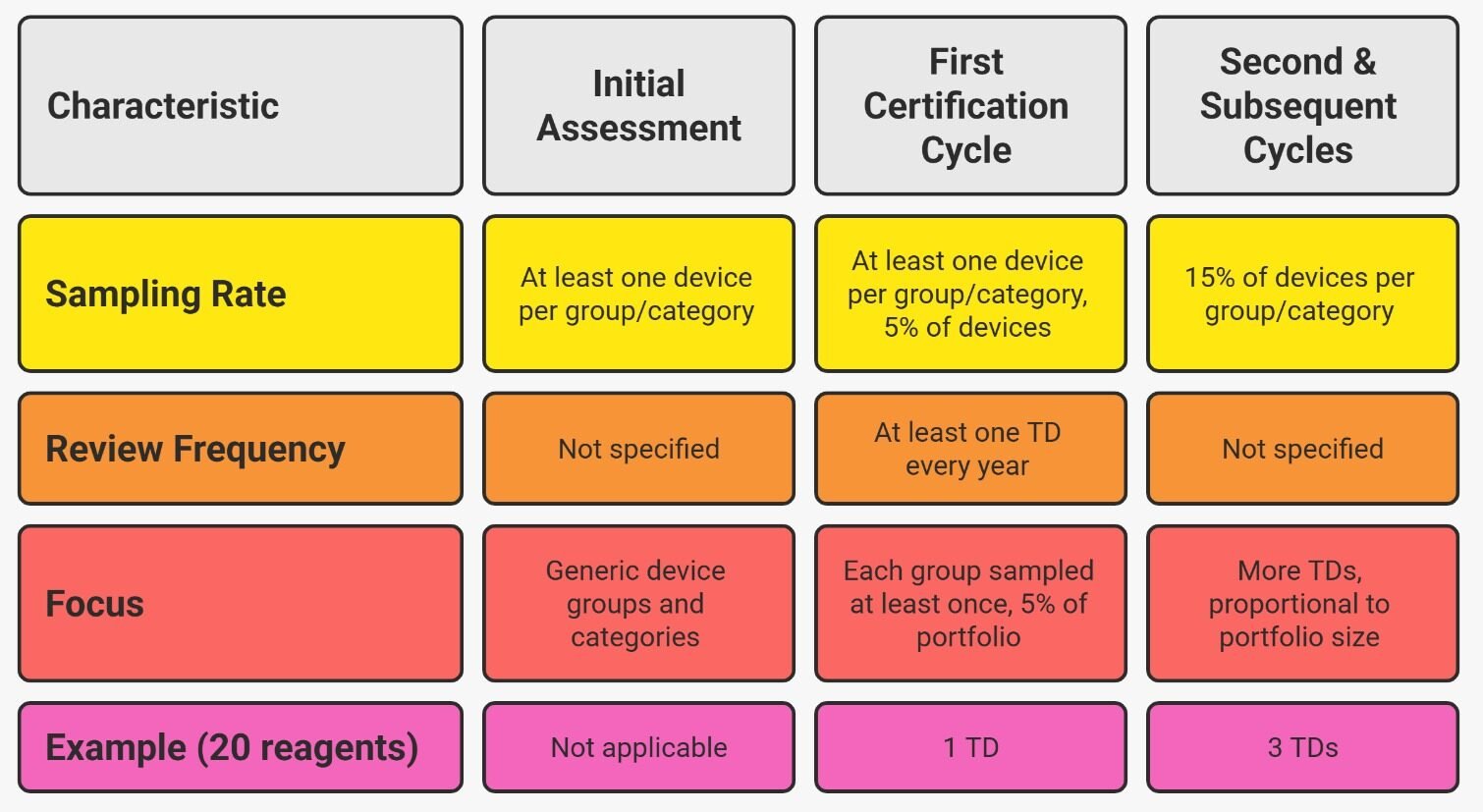
Before certification (Initial Assessment)– At least one device per generic device group and device category must be sampled before issuing the QMS certificate.
What it means: Your product portfolio must be grouped logically by:
- Device category (e.g., clinical chemistry, immunoassay, infectious disease).
- Generic device group within that category (e.g., lipid profile reagents, liver function reagents).
- The NB selects at least 1 TD from each group and category.
During surveillance within the first certification cycle:
During the first 5-year certification cycle, NB must:
- Sample at least one device per generic device group and device category
- Sample 5% of devices within each generic device group/category
- Review at least one TD every year
What this means in practice:
- If a generic device group contains 20 reagents, the NB will not review all 20, but 5% of them: 5% of 20 = 1 TD
- If another group contains 60 devices: 5% of 60 = 3 TDs
- However, NB does not need to repeat sampling of the same group if it was already reviewed during initial certification, unless risk or PMS triggers it.
Example: Suppose a manufacturer has 100 clinical chemistry reagents across 10 generic groups.
- NB already sampled 10 groups during certification
- During surveillance years:
- NB needs to ensure each group was sampled at least once
- Each year, NB must review at least 1 TD
- Total TDs during the full cycle = about 5% of portfolio, not all TDs
So, for 100 devices → 5% = 5 TDs over 5 years. Roughly 1 TD per year.
During Surveillance – Second & Subsequent Certification Cycles
Rule: Same principles as the first cycle, but sampling increases from 5% → 15% of devices per generic group/category.
What this means: Once certificate is renewed (2nd cycle):
- NB assesses more TDs because manufacturer has been on the market longer
- % increases for each generic group/category.
Example: If one generic group contains 20 reagents:
- First certification cycle: 5% = 1 TD
- Second certification cycle: 15% = 3 TDs
If the portfolio expands, sampling expands proportionally.
| Stage | % of TDs sampled | Minimum TDs per category/group | Minimum per year |
| Before Certification | Based on groups | 1 TD per generic group and device category | — |
| Surveillance – 1st Cycle (Years 1–5) | 5% | Ensure each group sampled once across cycle | ≥ 1 TD/year |
| Surveillance – 2nd & later cycles | 15% | Same group rule applies | ≥ 1 TD/year |
Importance of Annual Technical Documentation Updates
Annual updates are essential because:
-
New performance evaluation data must be incorporated
-
PMS and PMPF generate ongoing evidence every year
-
Analytical and clinical performance requirements evolve
-
New Common Specifications and standards may apply
-
Market feedback, complaints, and vigilance issues must be reflected
-
Version control and traceability are mandatory under IVDR
Regular updates reduce the risk of non-compliance during surveillance or sampling reviews.