Personalised Medical Devices
Personalised Medical devices are of two types (1) Adaptable Devices and (2) Patient Matched Medical Devices
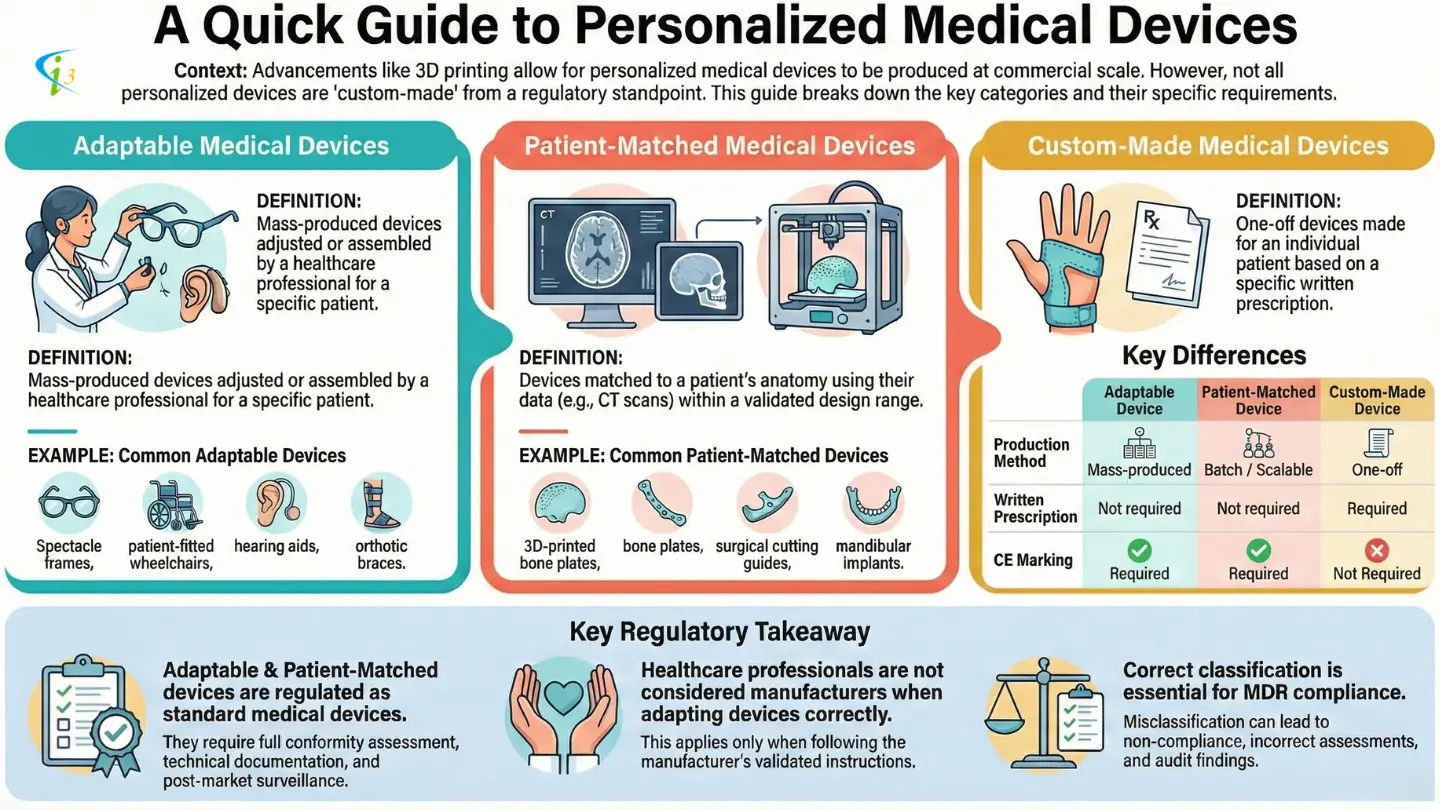
Advancements in technology have made it possible to manufacture individualized medical devices on a commercial scale, rather than through traditional artisanal methods. Techniques such as additive manufacturing (3D printing), using patient-specific data from CT scans, now enable the production of personalized medical devices that are not classified as custom-made medical devices. This article focuses on such personalized medical devices other than Custom-made Medical Devices, and clarifies their regulatory status.
Adaptable Medical Devices
Adaptable medical devices are mass-produced medical devices that require adaptation, adjustment, assembly, or shaping before use on a specific patient. These adaptations are performed at the point of care, traditionally by a healthcare professional, and must strictly follow the manufacturer’s validated instructions.
The purpose of such adaptations is to accommodate an individual patient’s anatomo-physiologic characteristics without altering the device’s intended medical function or safety profile.
Importantly, adaptable medical devices are not custom-made devices. They are produced on an industrial scale and are only individualized through predefined, controlled adjustments.
Common Examples of Adaptable Medical Devices. Typical examples of mass-produced adaptable medical devices include:
- Spectacle frames and optical glasses, where lenses and frames are assembled to form spectacles
- Patient-fitted wheelchairs, adjusted for posture, height, and mobility needs
- Hearing aids, including otoplastics and amplifiers customized for the patient
- Orthotic braces, shaped or adjusted to provide appropriate anatomical support
- Exo-prosthetics, assembled or adjusted to suit patient anatomy
In all these cases, the adaptation is expected and foreseen by the manufacturer as part of normal use.
Regulatory Position Under MDR Article 16(1)
Article 16(1) of the MDR provides clarity on the regulatory status of individuals who adapt these devices. It states that a person, such as a healthcare professional is not regarded as a manufacturer when they adapt, adjust, assemble, or shape an adaptable medical device for a particular patient, provided that:
- The actions are performed in accordance with the manufacturer’s validated instructions, and
- The adaptations do not modify the device in a way that affects compliance with applicable MDR requirements, and
- The intended purpose of the device remains unchanged.
This provision is crucial because it ensures that healthcare professionals can tailor devices to patients without unintentionally assuming manufacturer responsibilities, such as CE marking, technical documentation, or post-market surveillance obligations.
Patient-Matched Medical Devices
On the other hand, a Patient-Matched Medical Device is a medical device that meets all of the following criteria:
The device is matched to a patient’s anatomy within a specified design envelope, using techniques such as (a) scaling the device based on predefined anatomical references, or (b) incorporating full anatomical features derived from patient imaging data (e.g., CT or MRI scans)
- The device istypically produced in batches, or via a manufacturing process that is validated, repeatable, and reproducible;
- The device isdesigned and manufactured under the responsibility of a manufacturer, even if the design is developed in consultation with an authorized healthcare professional.
These devices leverage patient specific data while remaining within controlled and validated manufacturing parameters.
Common Examples of Patient Matched Medical Devices
- Plates used to fix a broken bone, which are made by 3D printing, based on a template model and DICOM files/ images of the patient. The plates are printed within the validated dimensional ranges allowed by the specified design envelope under the sole responsibility of the manufacturer.
- Cutting guides used in procedures such as knee arthroplasties, or guides used for pedicle screw placement, that are made by 3D printing based on MR or CT data to match a specific patient.
- Mandibular implants produced by a 3D printing manufacturer, from a template model and DICOM files.
- Made to order contact lenses which are produced on request typically in batches with validated or verified production processes using standardised tools and materials and within clearly specified dimensions. No specific or individual design process necessary.
- An externally worn orthosis to support, prevent or assist body functions, based on external 3D scan images and or measures, by a manufacturer who produces this under his sole responsibility, within validated parameters.
Distinction From Custom-Made Medical Devices
Patient-matched medical devices are not custom-made devices. The key differentiating factors include:
- Production method: Patient-matched devices are produced in batches or through mass production techniques, whereas custom-made devices are one-off products.
- Prescription requirement: Patient-matched devices do not require a written prescription by an authorised person, unlike custom-made devices.
- Design constraints: Patient-matched devices are created within a predefined design envelope, while custom-made devices are designed entirely outside standardised configurations.
This distinction has important regulatory consequences, particularly regarding conformity assessment routes and documentation requirements.
Regulatory Implications Under the MDR for Adaptable and Patient-Matched Medical Devices
Under the MDR framework, adaptable and patient-matched medical devices:
- Are regulated as standard medical devices, not custom-made devices
- Must undergo the applicable conformity assessment procedure based on their classification
- Require technical documentation, clinical evaluation, and post-market surveillance
- Must comply fully with General Safety and Performance Requirements (GSPRs)
A Comparative Summary Table
| Aspect | Custom-Made Medical Device | Adaptable Medical Device | Patient-Matched Medical Device |
|---|---|---|---|
| Production | One-off | Mass-produced | Batch / scalable |
| Patient Data Used | Yes | No | Yes |
| Design Envelope | No | Predefined | Predefined |
| Adaptation Location | Manufacturer | Point of care | Manufacturer |
| Written Prescription | Required | Not required | Not required |
| CE Marking | ❌ No | ✅ Yes | ✅ Yes |
| Manufacturer Responsibility | Shared with prescription | Manufacturer | Manufacturer |
| HCP Considered Manufacturer? | No | No (Art. 16(1)) | No |
| Regulatory Burden | Reduced | Full | Full |
Key Regulatory Takeaways
- Custom-made medical devices are truly bespoke and exempt from CE marking but require a written prescription and a custom-made statement.
- Adaptable medical devices are mass-produced and safely individualized at the point of care without changing regulatory accountability.
- Patient-matched medical devices combine personalization with industrial-scale control and remain fully under the manufacturer’s responsibility.
Correct classification is essential to avoid non-compliance, incorrect conformity assessment routes, or audit findings under the MDR.
Navigate MDR for Custom-Made Devices with Confidence
I3CGlobal delivers regulatory clarity for Annex XIII and high-risk implantables.