Post Market Clinical Follow Up (PMCF)!
Post market clinical follow up strategy defined in a manufacturer’s PMS and PMCF Plan is critical to staying in compliance with the Medical Device Regulation (MDR 2017/745). Contact us to understand how to plan a PMCF study and to find out what notified bodies, expect from the manufacturer.

Quick Contact
What is Post Market Clinical Follow Up?
Post Market Clinical Follow Up (PMCF) is a clinical subset of Post Market Surveillance (PMS) that focuses specifically on real-world clinical data related to safety, performance, and clinical benefits. The main purpose of PMCF is used to confirm clinical performance in real world use, detect rare or long-term adverse effects, adress clinical data gaps and validate assumptions made during CE approval
Post Market Clinical Follow Up activities must be as per article 61, establishes the legal obligation for manufacturers to perform and maintain a Clinical Evaluation for every medical device and annex XIV, Part B, which provides the detailed instructions on how to plan, perform, and document PMCF activities.
Under EU MDR 2017/745, manufacturers must maintain a continuous, proactive post-market system to ensure their medical devices remain safe, clinically effective, and compliant throughout their lifecycle. This system consists of PMS and PMCF
» PMS is mandatory for all devices.
» PMCF is mandatory unless a strong justification is provided.
Under EU MDR, PMS monitors your device, PMCF proves it clinically and both together protect your MDR CE mark .
Post Market Clinical Follow Up Under EU MDR
(Strategy, Data Collection, and the Consultant vs CRO Reality)
PMCF is no longer a easy activity under EU MDR 2017/745. For many manufacturers, PMCF has become one of the most scrutinized elements of the Clinical Evaluation lifecycle often determining whether a device retains its CE mark or faces serious Notified Body objections. A common point of confusion is who actually performs PMCF activities, especially data collection. Manufacturers frequently ask Can a regulatory consultant like I3CGLOBAL collect PMCF data for us? The answer lies in clearly understanding the division between regulatory consulting and operational execution.
| Activity | I3CGLOBAL – Regulatory Consultant | CMO / CRO – Operational Executor |
|---|---|---|
| PMCF Strategy | Defines scope, intensity, and compliance approach | Executes approved strategy |
| Site Selection | Advises on clinical requirments | Advises on type of hospitals and clinical settings
Physically visits sites and contracts investigators |
| Patient Recruitment | Defines inclusion/exclusion criteria | Recruits and tracks real patients |
| On-site Monitoring | Reviews monitoring outcomes | Conducts on-site Source Data Verification (SDV) |
| EDC Systems | Recommends compliant data approaches | Builds databases and manages raw data |
Special Note:
- I3CGLOBAL designs, governs, and documents PMCF.
- CROs collect, verify, and operationalize patient level data.
When a Separate CMO / CRO Is Mandatory for PMCF
If your PMCF Plan requires a Prospective Clinical Study, a regulatory consultant alone is not sufficient. In such cases, a dedicated CRO or strong internal clinical affairs team is essential to handle the following;
-
Ethics Committee submissions across hospitals
-
Real-time SAE and vigilance reporting
-
Source Data Verification (SDV)
-
On-site monitoring and investigator oversight
-
Multi-country operational logistics
I3CGLOBAL typically review the protocol, defines endpoints and statistics, prepares regulatory-ready documentation and coordinates with the CRO from a compliance perspective
When I3CGLOBAL Does Support PMCF Data Collection
Although I3CGLOBAL is not a traditional field CRO /CMO sending monitors to hospitals, they actively support several MDR accepted PMCF data sources, especially where operational burden is lower but regulatory rigor remains high.
- Literature based PMCF active clinical data collection from PubMed, Embase, and scientific databases, ongoing safety and performance trend analysis and fully aligned with MDR Annex XIV (Part B)
- PMCF Surveys & questionnaires related to design of MDR compliant physician and user surveys, Digital distribution and response tracking and Data aggregation and statistical interpretation
- Registry-Based PMCF for the Identification of relevant national or international registries, support with licensing and applicability assessment and clinical relevance and statistical justification for Notified Bodies
These methods are often sufficient for: Class I and IIa devices, Mature technologies and Devices with strong pre-market clinical evidence
Consultants deliver the “Technical File.” CROs deliver the “People, Patients, and Proof”
PMCF Considerations for Legacy Devices vs. New Devices under EU MDR
PMCF for Legacy Devices (MDD/AIMDD to MDR Transition)
Legacy devices are those that were CE marked under the MDD or AIMDD and are continuing or transitioning to compliance under EU MDR. For these devices, the primary objective of PMCF is to confirm continued safety and clinical performance in real-world use, identify any long-term or rare adverse events, and demonstrate that the benefit–risk profile remains acceptable under the stricter MDR framework. PMCF also plays a key role in addressing clinical evidence gaps identified during the MDR transition.
Notified Bodies reassess historical clinical data against MDR expectations, focusing on whether the original evidence was robust or largely equivalence-based, whether the clinical endpoints remain relevant, and whether the duration of follow-up is sufficient. Devices that relied heavily on equivalence under MDD typically face stronger PMCF requirements, as literature-only approaches are now rarely accepted. In such cases, Notified Bodies increasingly expect device-specific real-world clinical data.
PMCF for legacy devices must integrate complaint trending, vigilance and FSCA data, and post-market risk signals. Even when complaint rates are low, manufacturers must provide statistical justification demonstrating that the data adequately supports safety conclusions. In many cases, PMCF can be fulfilled without prospective clinical trials through a combination of literature reviews, PMS data analysis, user or physician surveys, and registry data, provided this approach is well justified. However, Class III and implantable legacy devices almost always require specific PMCF activities, often including formal studies.
During review, Notified Bodies typically challenge manufacturers to justify why existing PMCF remains sufficient, what has changed under MDR, and how continued clinical benefit is demonstrated.
PMCF for New Devices (First-Time MDR CE Marking)
New devices placed on the EU market under MDR are subject to significantly higher PMCF scrutiny, as there is no historical market experience to rely on. PMCF for new devices is intended to validate pre-market clinical assumptions, detect early safety signals, confirm intended clinical performance in routine use, and establish a robust real-world benefit risk profile.
For these devices, PMCF is viewed as a continuation of clinical investigation, rather than a maintenance activity, and is expected to begin immediately after market launch. The level of PMCF required increases with device risk class, novelty, and clinical uncertainty, with higher-risk and innovative devices often requiring structured, prospective PMCF studies.
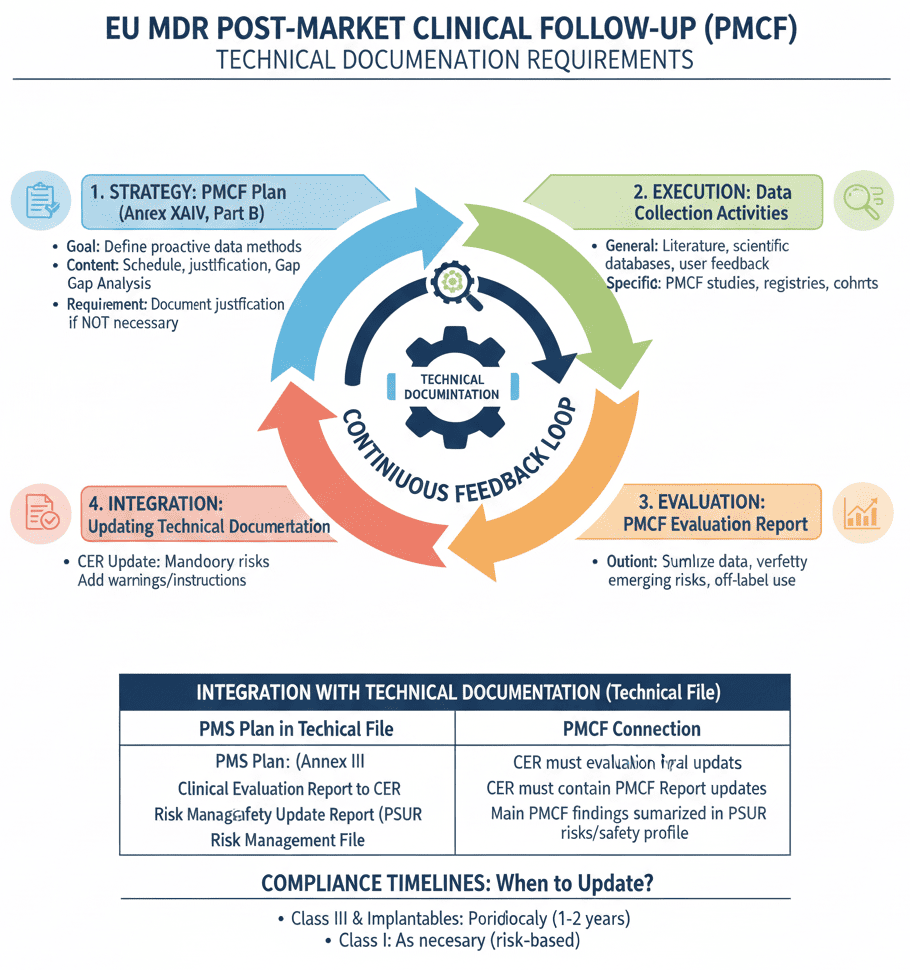
MDR 2017/745 PMCF Report for Medical Devices
Annex XIV Part B of the MDR contains detailed guidelines for the PMCF Plan. The post market clinical follow up must be carried out in accordance with a documented PMCF Plan, which must be presented as part of the medical device technical documentation. Findings from PMCF must be summarized on a regular basis in a PMCF Report.
The conclusion of the PMCF Report also becomes part of the device’s Clinical Evaluation Report. The contents of the Post market clinical follow up record are explained in MedDev 2.12/2 rev 2 even though it does not modify for MDR.
The important objectives of clinical follow up are the following
- Device Adverse events
- Severity and probability of occurrence of Side-effects
- Possible misuse of the device
- Safety and performance of the device in routine use
The above generated date is summarized and recorded. The report should also contain a summary of methods followed to collect the data. The manufacturer must justify the PMCF activities carried out is sufficient to address the objectives specified in the PMCF plan. The findings of the PMCF shall be analyzed by the manufacturer who shall document the results in a PMCF evaluation report. The PMCF evaluation report shall be part of the clinical evaluation report and the technical documentation.
Are you planning to obtain CE Certification through a Notified Body? Contact us. We specialize in Technical Documentation and Clinical Evaluation covering medical device PMCF.
Frequently Asked Questions
What are the conditions when above such studies might not be required?
- When the medium or long-term safety and clinical performance are already known from previous use of the device;
- Where other appropriate post-market surveillance activities would provide sufficient data to address the risks.
What are some of the cases that justify PMCF studies?
- Innovation, e.g., where the design of the device, the raw materials, the principles of operation, the technology, or the medical indications are novel;
- High-risk target populations e.g. pediatrics, elderly;
- High product-related risk e.g. based on design, materials, components, invasiveness, and clinical procedures;
- Identification of previously unstudied subpopulations which may show different benefit/risk ratio e.g. hip implants in different ethnic populations and so on.
State a few examples of methodologies to carry out PMCF studies?
- The extended follow-up of patients enrolled in premarket investigations;
- A new clinical investigation;
- A review of data derived from a device registry; or
- A review of relevant retrospective data from patients previously exposed to the device.
What is the use of the PMCF study data?
- The data and conclusions derived from the Post Market Clinical study are used to provide clinical evidence for the clinical evaluation. This may result in the need to reassess whether the device continues to comply with the General Safety and Performance Requirements (GSPR). Such assessment may result in corrective or preventive actions, for example, changes to the instructions for use/labelling, changes to manufacturing processes, revision of the device design, or public health notifications.
