Custom Made Medical Devices
Custom made medical devices (CMD) means any device specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications which gives, under that person’s responsibility, specific design characteristics, and is intended for the sole use of a particular patient exclusively to meet their individual conditions and needs.
Under Article 2(3) of Regulation (EU) 2017/745, a custom-made medical device is a device specifically manufactured in accordance with a written prescription issued by a qualified healthcare professional, which defines specific design characteristics and is intended for the sole use of an individual patient. Mass-produced devices, including those adapted or selected from standard designs, are explicitly excluded from the definition of custom-made devices.
MDR explicitly says below are NOT custom made devices:
“Mass-produced devices which need to be adapted to meet the specific requirements of any professional user and devices which are mass-produced by means of industrial manufacturing processes in accordance with the written prescriptions of any authorised person shall not be considered to be custom-made devices.”as per Article 2(3), second paragraph
The table below shows examples of device types, which might fall into the category of custom-made medical devices although some of the device types listed below will also be available as mass-produced, rather than custom-made medical devices.
| Device Type | Prescriber | Manufacturer |
| Dental appliances | Dentist | Dental laboratories |
| Artificial Eyes/Cosmetic Shells | Ocularist/Orbital Prosthetist | Ocularist or Ocular Technician |
| Maxillofacial Prosthesis | Medical Consultant or Prosthetist | Prosthetist |
| Hearing Aid Inserts/Molds | Medical Consultant, Audiology Technician or Hearing Aid Dispenser/Audiologist | Insert Maker |
| In-the-Ear Aids | Medical Consultant, Audiology Technician or Hearing Aid Dispenser/Audiologist | Aid Manufacturer |
| Orthopedic Footwear | Orthotist or Shoe fitter | Shoemaker |
| Joint Replacement Implants (designed for a specific individual) | Orthopedic Surgeon | Implant Manufacturer |
| Prosthetics and Orthotics | Rehabilitation Consultant, Orthopedic Consultant, Prosthetists or Orthotists | Prosthetic and Orthotic Service Companies and Manufacturers |
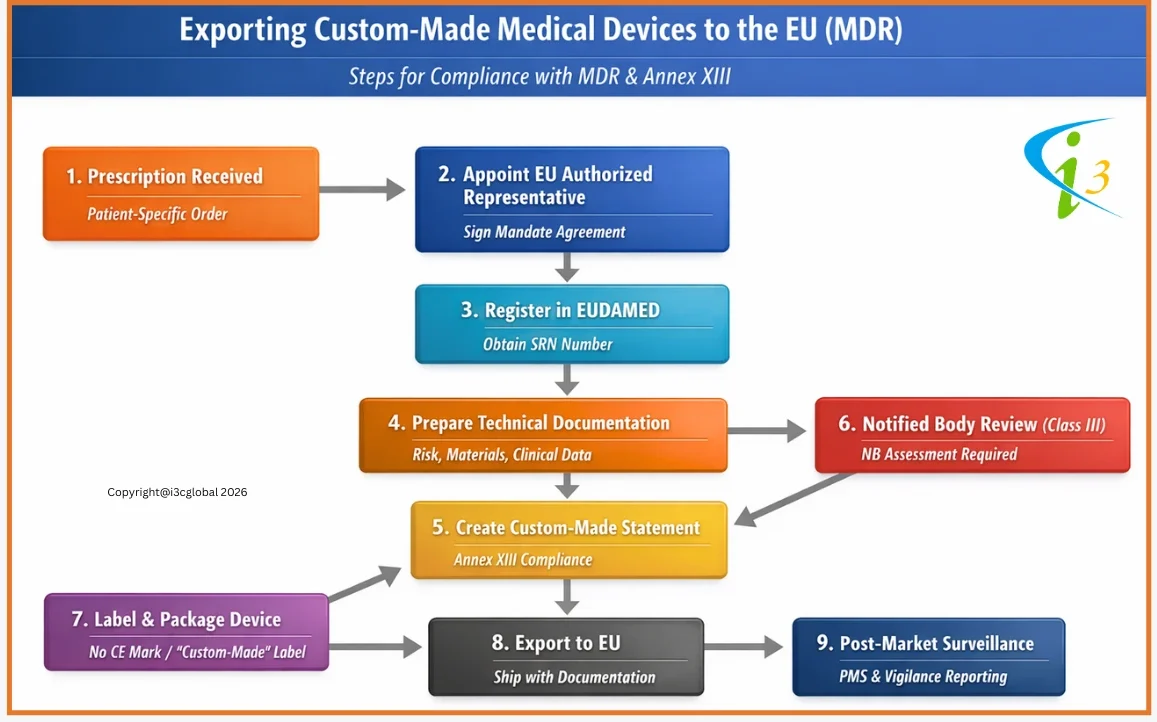
MDR CE Marking Procedure for Custom Made Devices
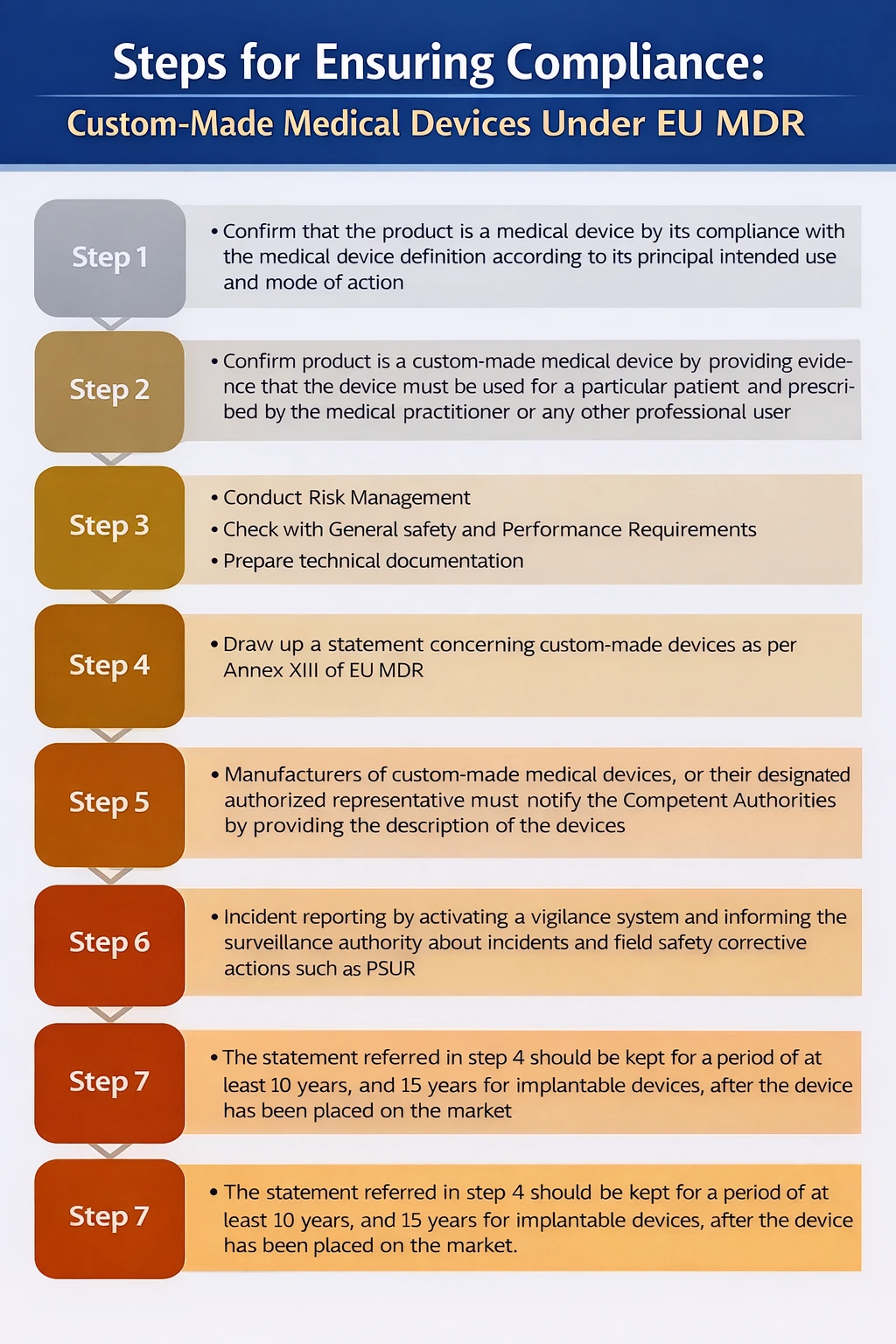
Annex XIII of the MDR is specifically designed to custom-made medical devices manufactures, while all non-custom-made devices require Technical Documentation (Annexes II and III of the MDR), including clinical evaluation, custom-made devices are exempt.
Custom-made medical devices manufactures must consider the traceability of the device throughout the design, manufacturing process, and performance of the device as per the intended use. The manufacturer is also obligated to follow post-production phase requirements.
MDR for Custom Made Class III Implants
Under EU MDR (Regulation (EU) 2017/745), custom-made Class III implantable devices are treated as high-risk. Manufacturers must comply with Annex XIII, which requires a specific custom made device statement, technical documentation, risk management, and clinical evaluation demonstrating conformity with the General Safety and Performance Requirements (Annex I). These statements must be retained for 15 years for implantable devices.
Unlike other custom made devices, Class III custom-made implants are not exempt from conformity assessment. Manufacturers must involve a Notified Body and undergo assessment under Annex IX, Chapter I (QMS route) or Annex XI, Part A (product conformity). Although CE marking is still not applied, Notified Body oversight, post-market surveillance, and vigilance obligations are mandatory due to the high-risk nature of these devices. No CE Certificate will be issued by NB.
⇒ You must have a signed contract with an NB by September 2024.
⇒ The full QMS certification must be in place by May 26, 2026.
What is NOT required for a custom-device manufacturer?

Navigate MDR for Custom-Made Devices with Confidence
I3CGlobal delivers regulatory clarity for Annex XIII and high-risk implantables.
