FDA Regulatory Pathway for high-risk Medical Devices -Premarket Approvals (PMAs)
The U.S. Food and Drug Administration (FDA) serves as the gatekeeper of medical device safety and effectiveness. Among its most stringent regulatory pathways is the Premarket Approval (PMA) process, which is mandatory for Class III medical devices—those that sustain or support human life, are implanted in the body, or pose a significant risk of illness or injury. Unlike the 510(k) pathway, which relies on demonstrating substantial equivalence to an existing device, PMA demands independent scientific evidence, often including clinical trials, to prove a device’s safety and efficacy.
What Is PMA and When Is It Required?
PMA is the FDA’s most rigorous regulatory mechanism, reserved for high-risk Class III devices. These include technologies such as implantable pacemakers, heart valves, artificial hearts, and deep brain stimulators. While some pre-amendment Class III devices may still qualify for 510(k) clearance, most new high-risk innovations must undergo the PMA process. This pathway is essential for novel devices that lack a predicate and could significantly impact patient outcomes. The PMA process ensures that such devices meet the highest standards before entering the market.
The PMA Review Process: Step-by-Step
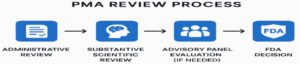
The PMA review process is a multi-phase journey designed to ensure that Class III medical devices meet the highest standards of safety and effectiveness before reaching patients. It unfolds in four key stages:
Why PMA Matters: For manufacturers, PMA approval is a badge of credibility, signaling that their device has undergone the most rigorous scrutiny. For healthcare providers and patients, it offers confidence in safety and effectiveness, backed by robust scientific evidence. While the process is demanding, it ensures that only the most thoroughly vetted technologies reach the market.
Administrative Review The process begins with an administrative review, where the FDA checks whether the PMA submission is complete and properly formatted. This includes verifying that all required sections—device description, clinical data, manufacturing details, labeling, and environmental impact—are present. If the application passes this initial screening, the FDA assigns a unique submission number and officially accepts it for substantive review.
Substantive Scientific Review Once accepted, the PMA enters the substantive review phase. Here, FDA experts—including biomedical engineers, statisticians, and clinical reviewers—conduct a deep dive into the data. They assess the validity of clinical trial results, examine non-clinical testing outcomes, and evaluate the device’s design and manufacturing protocols. The reviewers also perform a risk-benefit analysis to determine whether the device’s potential advantages outweigh any associated risks.
Advisory Panel Evaluation (If needed) For particularly novel or complex devices, the FDA may convene an advisory panel composed of independent experts in medicine, science, and engineering. This panel reviews the application and provides recommendations based on their expertise. While the panel’s input is not binding, it often plays a critical role in shaping the FDA’s final decision, especially for devices with significant public health implications.
For a detailed proposal with a Statement of Work, please complete the Request for Quote (RFQ) form provided separately for FDA 510(k) and IVDR CE Marking for
FDA Decisions
After completing the scientific and advisory evaluations, the FDA issues a formal decision. There are several possible outcomes:
⇒ Approval: The device is deemed safe and effective, and may be marketed in the U.S.
⇒ Approvable Letter: The device is likely to be approved pending minor changes or additional information.
⇒ Not Approvable Letter: The application lacks sufficient evidence or raises safety concerns.
⇒ Request for Additional Information: The FDA may ask for further data before making a final determination.
The entire review process typically spans 9 to 18 months, depending on the complexity of the device and the quality of the submission. Manufacturers should also be prepared for post-approval requirements, such as continued safety monitoring, periodic reporting, and possibly additional clinical studies.
PMA Application Contents
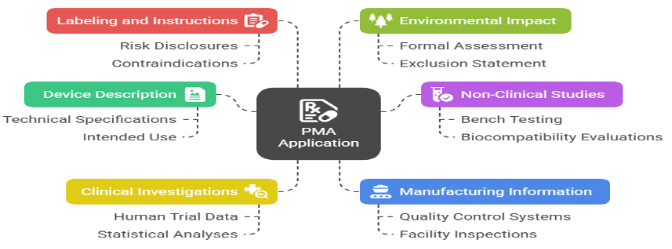
A PMA submission is a comprehensive dossier. A successful PMA submission includes:
⇒ Device Description: Technical specs, design, and intended use
⇒ Non-Clinical Studies: Bench testing, biocompatibility, software validation
⇒ Clinical Data: Human trials with statistical analysis
⇒ Manufacturing Information: Quality systems and facility details
⇒ Labeling: Instructions, warnings, and patient information
⇒ Environmental Impact Statement: As required under 21 CFR Part 814
Recent Trends and Updates as of October 2023, the FDA mandates that all 510(k) submissions be filed electronically via eSTAR, and PMAs are increasingly submitted through the CDRH Portal. The FDA also maintains a searchable PMA database, which lists approved devices, manufacturers, and approval dates—an invaluable resource for industry stakeholders.
PMA and Other Regulatory Pathways
Understanding the strategic differences between PMA and other FDA pathways is essential. The 510(k) pathway is designed for Class I and II devices that can be shown to be substantially equivalent to an existing legally marketed device. It is faster, less expensive, and suitable for low- to moderate-risk products, but it does not require independent proof of safety and effectiveness.
The De Novo pathway caters to novel, low- to moderate-risk devices that lack a predicate but do not meet Class III risk criteria. It allows manufacturers to establish a new device classification and predicate for future 510(k) submissions. In contrast, PMA is the gold standard for high-risk, life-sustaining technologies, offering unmatched regulatory credibility, market exclusivity, and global recognition.
Final Thought
Premarket Approval is more than a regulatory hurdle—it is a testament to scientific rigor, patient safety, and technological innovation. For manufacturers, achieving PMA status validates their product’s reliability and clinical value. For regulators, it ensures that only the most thoroughly vetted devices reach the market. And for patients, it offers peace of mind that their health is protected by the highest standards of oversight. In today’s rapidly evolving medical landscape, PMA remains the benchmark for excellence. It challenges innovators to rise above the norm, prove their claims with data, and deliver solutions that truly make a difference. Whether you’re a startup developing a novel implant or a global company refining life-saving technology, PMA is your path to credibility, impact, and trust.