Quick Contact
Leading EU Representative for Medical Device providing EU Registration Service for Non European Medical Device Manufactures

EUDAMED Registration
EUDAMED Registration is a European Database an IT system proposed by the European Commission for medical devices and in-vitro diagnostic devices to meet certain requirements mentioned in regulations EU MDR 2017/745 and EU IVDR 2017/746, respectively.
This registration will enhance the transparency for the public and healthcare professionals and coordination of information regarding medical devices and IVDs on the EU market. As per the information on the EU website, the EUDAMED is structured around 6 interconnected modules and a public website:
♦ Actors Registration
♦ UDI/Devices Registration
♦ Notified Bodies and Certificates
♦ Clinical Investigations and Performance Studies
♦ Vigilance and Post Market Surveillance
♦ Market Surveillance
It is known from the EU website that the Actor registration module (first module) will be available first and its deployment is expected by December 2020, the second and the third modules are expected to be made available by May 2021.
EUDAMED Device Registration Enforced Now!
EUDAMED Registration is now mandatory under the EU Medical Device Regulation (MDR 2017/745) and the In Vitro Diagnostic Regulation (IVDR 2017/746). However, the full implementation of all modules of EUDAMED is delayed.
⊗ The “Actor Registration” module is fully operational and mandatory. All manufacturers, authorized representatives, importers, and system/procedure pack producers need to register in EUDAMED to obtain a Single Registration Number (SRN).
⊗ Other modules, such as UDI/Device Registration, will become mandatory after the full implementation of the database, which is expected by 2026.
While a few modules are already required, complete compliance with European database on medical device is expected once all modules are fully functional.
Do you need an email containing full details within 2 minutes?
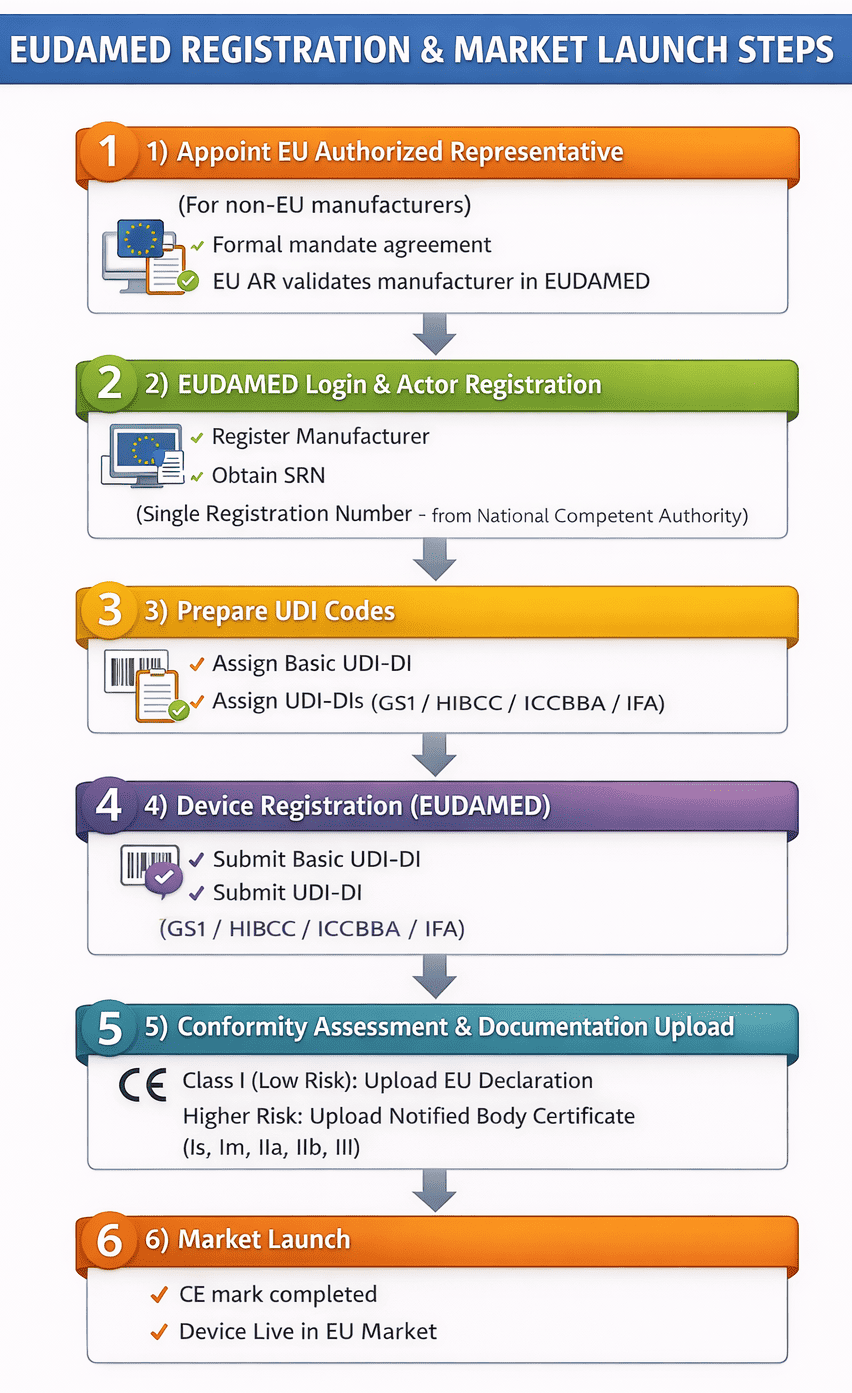
Steps for EUDAMED Registration!
Once the technical documentation is finalised and the EU Authorised Representative is appointed (where required), manufacturers can initiate the EUDAMED registration process a mandatory step for placing Medical Devices and IVDs on the European market under EU MDR & IVDR.
This process applies to all device risk classes and must be completed before engaging a Notified Body for conformity assessment, where applicable.
Step 1: Obtain Basic UDI-DI & UDI Codes
Every EUDAMED registration begins with proper device identification followed by manufacturers must obtain:
-
Basic UDI-DI
-
UDI-DI(s) for each device and packaging level
These identifiers are issued by EU-designated issuing entities, including GS1, IFA GmbH, HIBCC and ICCBBA. These identifiers form the backbone of device traceability and regulatory transparency.
Step 2: Actor Registration & SRN Assignment
All economic operators(Manufactures, EAR, Importer) involved in placing a device on the EU market must follow this step. After submission and verification by the National Competent Authority, each operator is issued a Single Registration Number (SRN). The SRN is mandatory for Device registration and Notified Body applications.
Step 3: Device Registration in EUDAMED
With the SRN in place, manufacturers proceed to the UDI/Device Registration Module by submitting Device Information and Manufacturer details along with below information:
-
Basic UDI-DI and UDI-DI
-
Packaging and quantity details
-
Member State information
This step officially links your device to your organization in EUDAMED.
Step 4: Conformity Assessment & CE Marking
-
Low-risk devices (Class I non-sterile / non-measuring): Manufacturers must prepare and maintain the EU Declaration of Conformity in line with the applicable EU regulations to demonstrate compliance prior to market placement.
-
Higher-risk devices (Class Is, Im, IIa, IIb, III): A conformity assessment by a designated Notified Body is required, and the corresponding Notified Body certificates must be uploaded to EUDAMED; upon successful assessment, CE marking approval is granted, allowing the device to be placed on the EU market.
Step 5: Market Launch
Once all regulatory requirements are met your device is now legally placed on the EU market
-
EUDAMED registration completed
-
CE marking obtained
-
EU Declaration of Conformity finalised
Post-market surveillance and vigilance obligations then begin as per MDR and IVDR.
One partner. One process. Full compliance. As your EU Authorized Representative, we handle EUDAMED registration from start to market launch.
We are a European Authorized Representative for Medical Devices and IVD,s. I3CGlobal also provide EUDAMED Registration for all our Foreign manufacturers. Contact us for more details and pricing.
Frequently Asked Questions
When should EUDAMED registration be completed in the regulatory process?
EUDAMED registration should be completed after technical documentation is prepared and before applying to a Notified Body for conformity assessment, where applicable. Completing EUDAMED registration early helps avoid delays in CE marking and ensures a smoother path to EU market launch.
What are the documents required for EUDAMED registration?
The following are the documents required:
- EAR Agreement (For manufactures Only)
- EAR Mandate (For manufacturers Only)
- Declaration of Conformity
- Manufactures Declaration
- PRRC details
Is appointing an EU Authorized Representative mandatory?
Yes. Non-EU manufacturers must appoint an EU Authorized Representative before initiating EUDAMED registration. The EAR acts as the legal contact within the European Union and is responsible for supporting regulatory compliance, including validation of the manufacturer’s actor registration in EUDAMED.
EUDAMED Registration Timeline?
The average timeline is 2 to 3 weeks.
Does UDI updating a must now?
Once the UDI/Device Registration module in EUDAMED becomes fully operational, manufacturers will need to update their device information in the system, including the UDI. Until then, UDI updates may be handled through national regulatory systems or databases, depending on the Member State’s provisions.