EU MDR Medical Device Classification
This page provides a concise overview of the medical device classification system under the MDR 2017/745 regulation. It offers examples of devices within each class and indicates the corresponding type of certification required. This information is intended to provide a quick reference for website viewers seeking to understand the EU MDR classification process. Website visitors are advised to refer left side panel for more information about individual risk class.
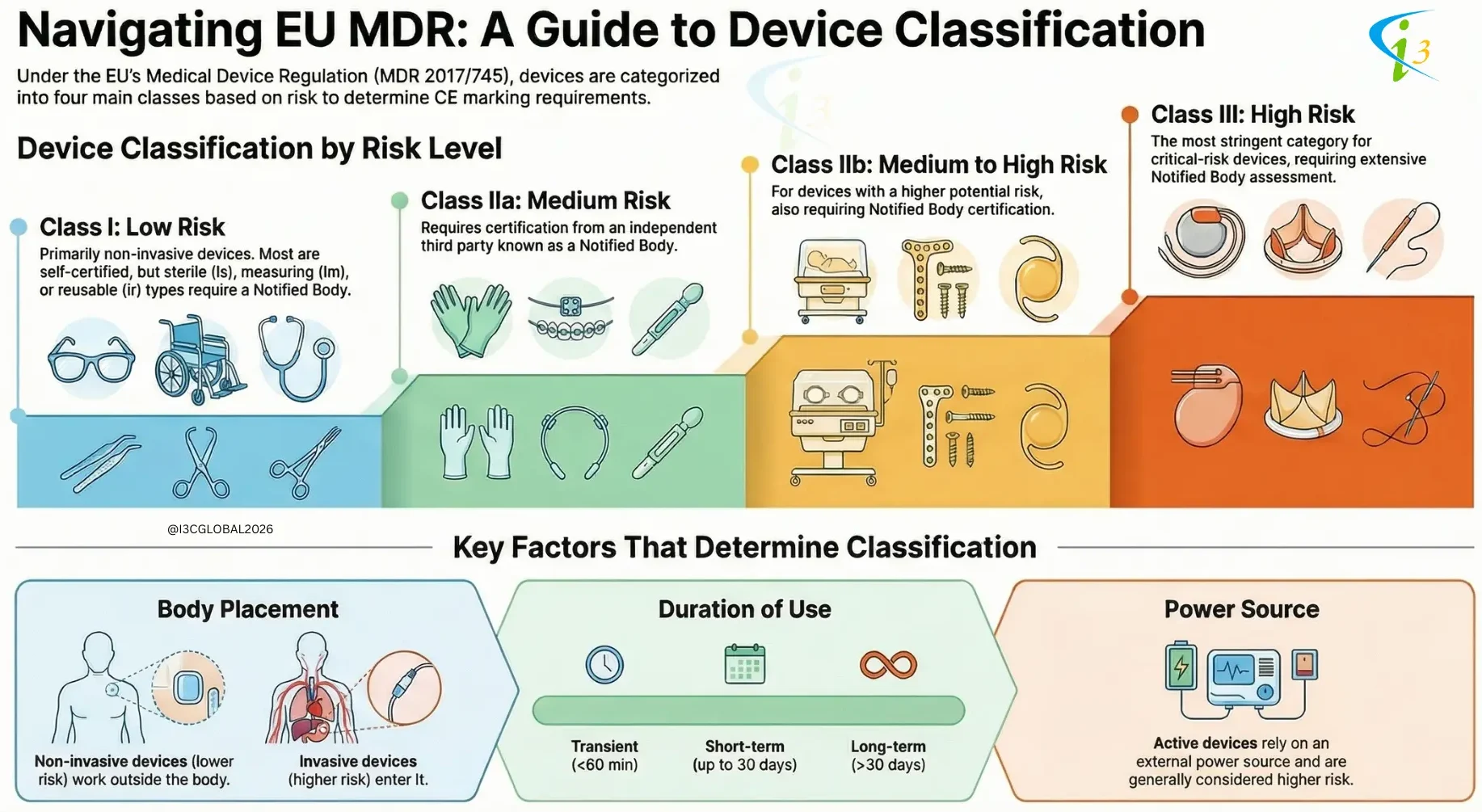
The Medical Device Regulation (MDR) 2017/745 according to article 51 categories medical devices into four classes based on their risk level: Class I, Class IIa, Class IIb, and Class III. The classification determines the conformity assessment route a manufacturer must take to place a device on the market.
The determination of the Medical device classification and the corresponding rule is the first step in the CE marking process. Based on the class and the rule of the device, the technical file and the Notified Body application can be filed. In the new MDR, there are now 22 rules in Annex VIII.
The classification of medical devices is a critical process that ensures patient safety and efficacy. By considering factors such as invasiveness, body placement, and reliance on external power, regulatory bodies can assign appropriate risk classification and implement corresponding regulatory controls. This framework helps to balance innovation with patient protection, ensuring that medical devices are safe and effective for their intended use.

(Listen for 7 Minutes)
EU MDR Medical Device Classification Based on Invasiveness and Body Placement
Medical devices are classified based on their potential risk to patients, which is heavily influenced by their invasiveness and interaction with the body.
Non-Invasive Devices:
-
These devices operate externally, without penetrating the body’s surface or entering any body openings.
-
Due to their limited interaction with the body’s internal systems, they generally pose lower risks.
-
Consequently, non-invasive devices are typically classified as either Class I or Class IIa.
Invasive Devices:
-
Invasive devices are designed to penetrate the body, either by piercing the skin or entering through a natural body opening.
-
This direct interaction with internal tissues and systems increases the potential for harm, leading to a higher risk profile.
-
As a result, invasive devices are generally classified as either Class IIb or Class III.
EU MDR Classification Based on Active Device
-
Active devices rely on an external power source to function. This power source can be electricity, batteries, or chemical reactions.
-
The reliance on external power introduces additional risks, such as malfunction, power failure, or unintended energy delivery.
-
Active devices are generally classified as either Class IIb or Class III.
EU MDR Classification Based on Duration of Use
Medical devices are classified based on the duration for which they are intended to be in continuous use. The three primary classification are:
1. Transient Devices: Transient devices are those intended for continuous use for a period of less than 60 minutes.
Examples are Hypodermic needles used for injections, Surgical drapes used during short procedures, Briefly used diagnostic probes, Tourniquets applied for short intervals and Single-use examination gloves.
Considerations:
-
-
These devices typically pose a lower risk of infection or adverse reactions due to the short duration of contact.
-
Material selection focuses on functionality and ease of use for the brief period of application.
-
Sterilization and disposal protocols are essential to prevent cross-contamination.
-
2. Short-Term Use Devices: Short-term use devices are intended for continuous use for a period between 60 minutes and 30 days.
Examples are Urinary catheters used post-surgery, Wound dressings applied for healing, External fixation devices used for fracture stabilization, Contact lenses worn daily and Electrodes used for short-term monitoring (e.g., ECG).
Considerations:
-
-
Material biocompatibility is more critical than for transient devices, as prolonged contact increases the risk of adverse tissue reactions.
-
Device design must consider patient comfort and ease of use over the specified duration.
-
Cleaning and maintenance protocols may be necessary for reusable short-term devices.
-
Risk of infection must be carefully managed through appropriate sterilization or disinfection procedures.
-
3. Long-Term Use Devices: Long-term use devices are intended for continuous use for a period more than 30 days.
Examples are Implantable pacemakers, Artificial joints, Intraocular lenses, Drug-eluting stents and Hearing aids.
Considerations:
-
-
Biocompatibility is paramount, as the device will be in constant contact with body tissues for an extended period.
-
Material durability and resistance to degradation are critical to ensure long-term functionality.
-
Device design must minimize the risk of complications such as infection, inflammation, or device failure.
-
Rigorous testing and clinical trials are required to demonstrate safety and efficacy over the intended lifespan of the device.
-
Post-market surveillance is essential to monitor long-term performance and identify any potential issues.
-
Interested to know more about the EU MDR medical device classification and our CE Marking services? We will send your mailbox directly. Privacy Policy >
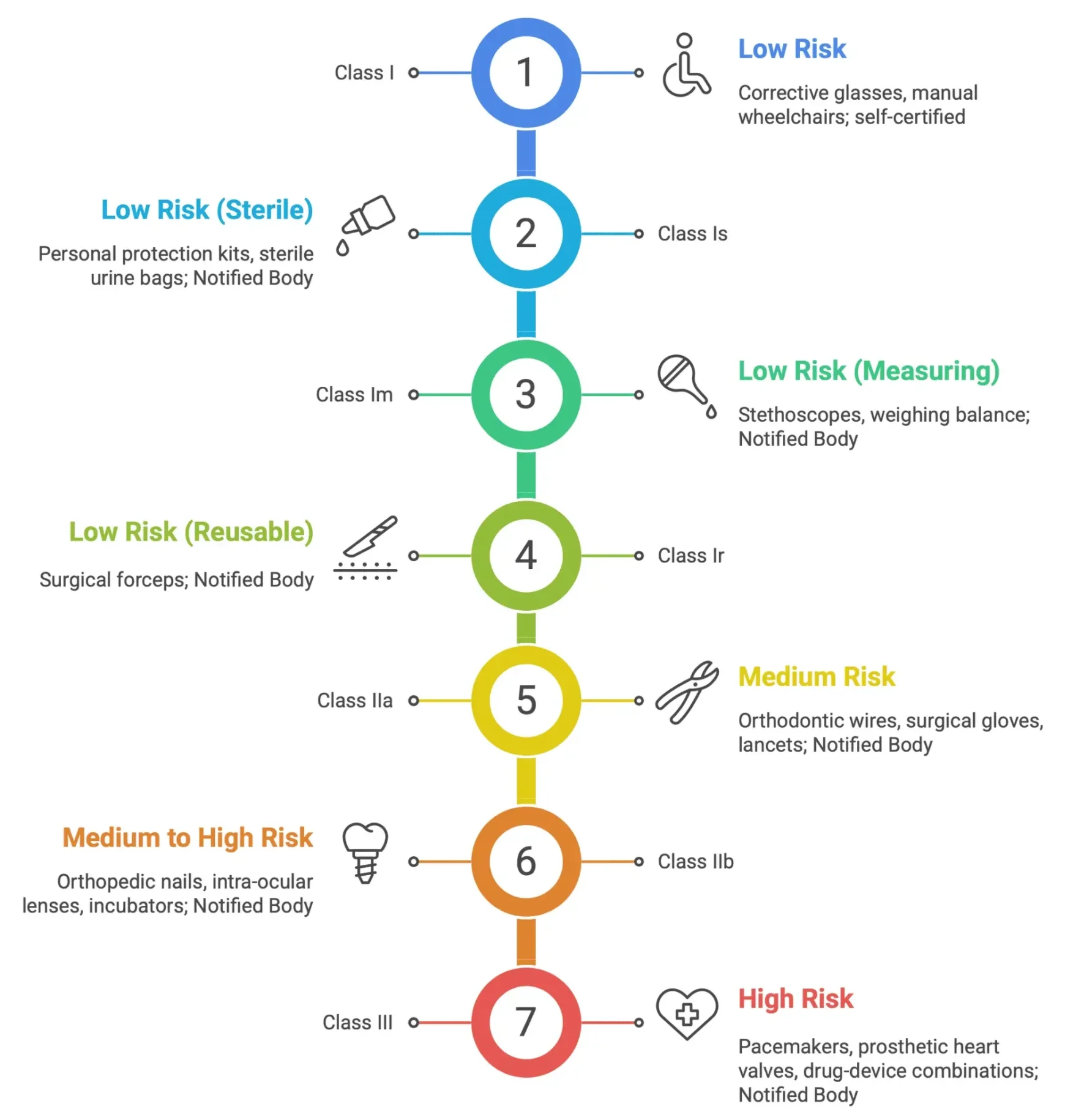
Medical Device Classification Examples
I3CGLOBAL completed 300+ CE certifications worldwide by supporting manufacturers with Medical device classification of simple and complex device technologies for faster-notified body approval. We have offices in India, Europe, and the USA to help with classification and technical documentation.
Medical device classification Rules
The classification of medical devices under the EU MDR is based on the potential risk associated with their use. Annex VIII provides a set of rules to guide manufacturers in determining the appropriate classification for their devices. These rules are categorized based on the type of device (non-invasive, invasive, active) and specific characteristics related to their intended use and duration of contact with the body.
Rules 1–4: Non-Invasive Devices
These rules primarily address devices that do not penetrate the body surface or enter internal body cavities. Non-invasive devices are generally classified as Class I. This applies to devices that come into contact with intact skin only.
- Class IIa:Devices intended for channeling or storing blood, body liquids, cells or tissues, liquids or gases for eventual infusion, administration or introduction into the body. Devices that modify the biological or chemical composition of human tissues or cells. Devices that aid in wound healing.
- Class IIb: Devices used for disinfecting medical devices. Devices intended for recording diagnostic images generated by X-ray, MRI, or ultrasound.
- Class III: Devices that come into contact with injured skin and are intended to be used where there is a breach of the skin and which heal by secondary intent.
Rules 5–8: Invasive Devices
These rules cover devices that penetrate the body, either through a body orifice or through the surface of the body. Invasive devices tend to be classified as Class IIa, Class IIb, or Class III, reflecting the increased risk associated with penetrating the body’s natural barriers.
- Class IIa: Devices intended for short-term use (less than 30 days) that are surgically invasive. Devices intended for short-term use that are intended to administer medicinal products by inhalation.
- Class IIb: Devices intended for long-term use (more than 30 days) that are surgically invasive. Devices intended to administer medicinal products by inhalation. Devices intended to come into contact with injured skin.
- Class III: Devices intended to come into direct contact with the heart, central circulatory system, or central nervous system. Devices that incorporate a medicinal substance. Implantable devices.
- Class I:Reusable surgical instruments.
Rules 9–13: Active Devices
These rules apply to devices that rely on a source of electrical energy or any source of power other than that directly generated by the human body or gravity. Active devices can fall into Class I, Class IIa, Class IIb, or Class III, depending on factors such as their potential hazard, body placement, and duration of use.
- Class I: Therapeutic devices that do not pose a significant risk.
- Class IIa: Therapeutic devices that deliver energy in a potentially hazardous way. Diagnostic devices that supply energy to the body.
- Class IIb: Devices intended to control, monitor, or directly influence the performance of active implantable medical devices. Active devices intended for administering medicinal products, body liquids or other substances to the body.
- Class III: Active devices intended to emit ionizing radiation. Active devices intended to support, modify, replace or restore biological functions or structures.
Rules 14–22: Special Rules
These rules address specific types of devices or situations that require special consideration. They cover a wide range of devices, including:
-
Devices incorporating a medicinal substance.
-
Devices utilizing non-viable animal or human tissues or cells.
-
Devices manufactured utilizing derivatives of human blood or plasma.
-
Contraceptive or prevention of sexually transmitted diseases devices.
-
Devices specifically intended for cleaning, disinfecting or sterilizing medical devices.
-
Devices for recording diagnostic images generated by X-ray.
-
Devices manufactured utilizing nanomaterials.
These rules often lead to higher classifications (Class IIb or Class III) due to the increased risk associated with these specific characteristics. For example, devices incorporating a medicinal substance are generally classified as Class III. Devices utilizing non-viable animal or human tissues or cells are generally classified as Class III, except if the tissues or cells are manufactured to be non-viable or are devitalized.
What is special in EU MDR Classification?
EU Regulation 2017/745 represents a significant overhaul of the European Union’s regulatory framework for medical devices. One of the most notable changes introduced by the MDR is the inclusion of certain aesthetic devices without an intended medical purpose under the definition of medical devices. This change is codified in Annex XVI of the MDR, which lists specific categories of devices that are now subject to the same regulatory requirements as traditional medical devices.
The inclusion of these devices in the MDR reflects a growing recognition that even devices without a direct medical purpose can pose significant risks to patients if they are not properly designed, manufactured, and used.
Devices Included in Annex XVI of the MDR specifically identifies the following categories of devices as medical devices, even if they do not have an intended medical purpose:
1. Different Kinds of Lenses: This category includes all types of lenses intended for placement in the eye, including both prescription and non-prescription lenses. Of particular note is the inclusion of “fun lenses” and non-prescription colored contact lenses. These lenses, often sold for cosmetic purposes, can pose significant risks to eye health if they are not properly fitted and cared for. Risks include corneal abrasions, infections, and even vision loss.
2. Implants without a Medical Function: This category includes implants intended for cosmetic purposes, such as breast implants. While breast implants with a medical indication (e.g., for reconstruction after mastectomy) were already regulated as medical devices, this provision extends the regulation to breast implants used solely for aesthetic augmentation. This reflects concerns about the long-term safety and potential complications associated with breast implants, such as capsular contracture, rupture, and the potential link to certain types of cancer.
3. Cosmetic Fillers: This category includes substances intended to be injected into the skin or mucous membranes for cosmetic purposes, such as lip augmentation or wrinkle reduction. Cosmetic fillers have become increasingly popular in recent years, but they are not without risks. Potential complications include allergic reactions, infections, granulomas, and vascular occlusion, which can lead to tissue necrosis and even blindness.
4. Devices for Liposuction, Lipolysis, and Lipoplasty: This category includes devices and instruments used for procedures such as liposuction (surgical removal of fat), lipolysis (non-surgical fat reduction), and lipoplasty (surgical reshaping of fat). These procedures can carry significant risks, including bleeding, infection, nerve damage, and skin irregularities.
5. Light-Emitting Devices: This category includes devices that emit light, such as lasers and intense pulsed light (IPL) devices, used for hair removal, tattoo removal, skin resurfacing, and other cosmetic treatments. These devices can cause burns, scarring, changes in skin pigmentation, and even eye damage if they are not used properly.
6. Brain Stimulation Devices: This category includes devices intended to stimulate or influence brain activity for cosmetic or lifestyle purposes, such as mood enhancement or cognitive improvement. While the use of brain stimulation devices for medical purposes is well-established, the use of these devices for non-medical purposes is a relatively new and rapidly evolving field. The long-term safety and efficacy of these devices are not yet fully understood, and there are concerns about potential risks such as seizures, cognitive impairment, and psychological effects.
Frequently Asked Questions
What are Special Rules?
Special rules cover devices incorporating medicinal products, devices manufactured utilizing tissues or cells, and devices for contraception or prevention of the transmission of sexually transmitted diseases. These rules falls in 14 to 22 and occupationally classified as Class IIa, Class IIb, or Class III
What are the new class included in EU 2017/745?
The New Medical device classification included the reusable surgical instruments category, which applies to all surgical instruments sold by manufacturers in non-sterile conditions and sterilized by hospitals post-usage. These are multi-use devices.