A Guide to Medical Device Relabeling in the EU
Navigating the complexities of the European Union’s Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) can be challenging, especially for economic operators involved in importing, distributing, or rebranding devices. A critical pitfall often overlooked is Article 16 of the MDR/IVDR, which can unexpectedly elevate a distributor or importer to the full legal responsities of a manufacturer. This guide will clarify when this occurs and how to maintain compliance.
The Article 16 Trigger: When Your Brand Becomes Your Burden
At its core, Article 16 states that if an importer, distributor, or any other economic operator places a device on the EU market under their own name, trade name, or registered trademark, they automatically assume the obligations of a manufacturer. This holds true unless a formal agreement explicitly designates the original manufacturer as solely responsible for MDR/IVDR compliance.
This means that simply changing a device’s branding, even if manufactured by an Original Equipment Manufacturer (OEM), can lead to significant new regulatory responsibilities.
Understanding Your Role: Distributor, OEM, or Manufacturer?
Your legal status under the MDR/IVDR depends heavily on your activities regarding the device’s branding and market placement. The table below illustrates common scenarios:
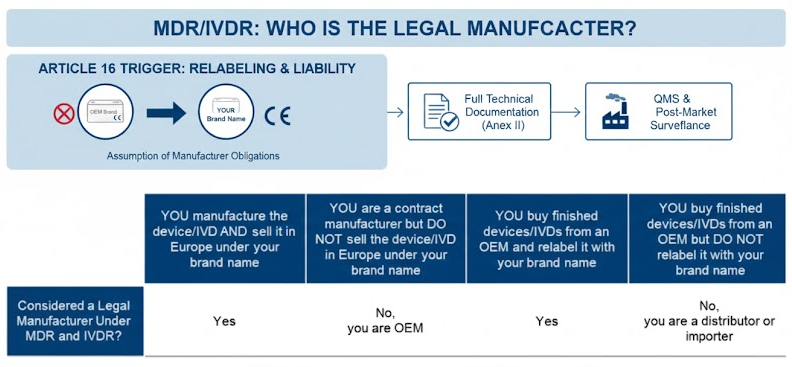
-
You Manufacture & Sell Under Your Brand: Clearly a legal manufacturer.
-
You are a Contract Manufacturer (OEM): Not the legal manufacturer unless you also sell under your own brand in the EU.
-
You Buy Finished Devices & Relabel with Your Brand: This is the critical trigger point. You become the legal manufacturer.
-
You Buy Finished Devices & Do NOT Relabel: You remain a distributor or importer, with responsibilities for verification, but not full manufacturer obligations.
For a detailed proposal with a Statement of Work, please complete the Request for Quote (RFQ) form provided separately for FDA 510(k) and IVDR CE Marking for any device
The Burden of Proof: Performance and Biocompatibility Testing
One of the most significant implications of becoming a legal manufacturer is the responsibility for demonstrating the device’s safety and performance. This includes, but is not limited to, performance testing and biocompatibility testing.
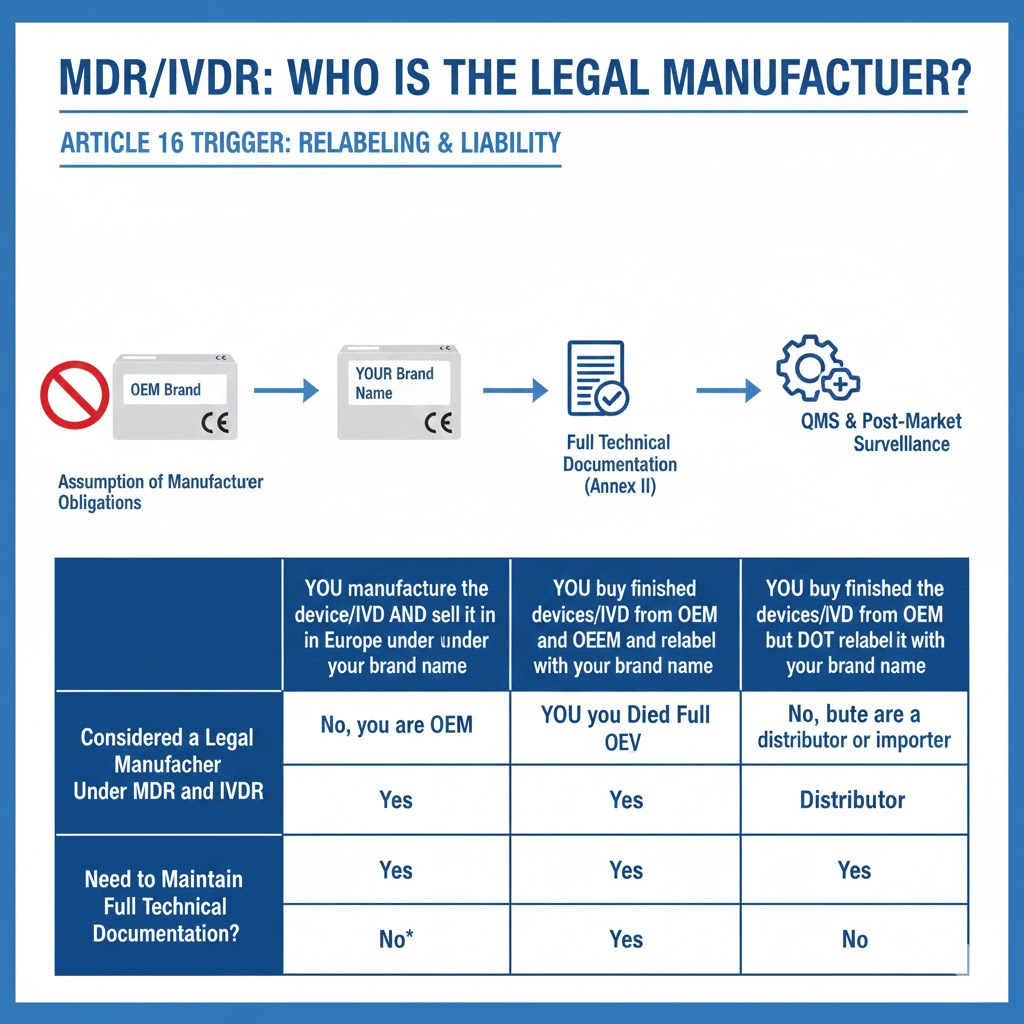
If you are the Legal Manufacturer (by relabeling or original manufacture)
-
Full Responsibility: You are accountable for ensuring the device meets all General Safety and Performance Requirements (GSPRs).
-
Performance Testing: You must ensure that all necessary bench testing, simulated use, and clinical evaluations are conducted to prove the device’s intended performance and safety.
-
Biocompatibility Testing: You must demonstrate the biological safety of all patient-contacting materials, typically by adherence to ISO 10993 standards and maintaining comprehensive test reports.
-
Technical Documentation: All original test protocols, raw data, and analyses must be part of your Technical File (MDR Annex II). Even if leveraging OEM data, you must have full access and control over it.
If you are a Distributor/Importer (without relabeling or with an explicit agreement)
-
OEM Responsibility: The Original Equipment Manufacturer (OEM) remains the legal manufacturer and is solely responsible for conducting and documenting these tests as part of their CE marking process.
-
Your Verification Role: Your primary obligation is to verify that the device is correctly CE marked and that the OEM has fulfilled their manufacturer duties. You are not required to conduct new tests or hold the full technical documentation.
The Technical File Burden: What You Need to Hold
The moment you assume manufacturer status, the requirement to maintain complete Technical Documentation (as per MDR Annex II) becomes yours. This is a substantial undertaking.

Your Technical File must include:
-
Device description and specification, including variants.
-
Information to be supplied by the manufacturer (labels, Instructions For Use).
-
Information on the design and manufacturing.
-
General Safety and Performance Requirements (GSPR) solutions.
-
Benefit-risk analysis and risk management (ISO 14971).
-
Verification and validation reports (e.g., performance and biocompatibility per ISO 10993).
-
Pre-clinical and clinical data (Clinical Evaluation Report – CER).
-
Post-market surveillance plan and Post-Market Clinical Follow-up (PMCF) plan.
This documentation must be maintained and kept up-to-date for at least 10 years after the last device is placed on the market (15 years for implantable devices).
Mitigating the Risk: The Private Label Agreement
To avoid inadvertently becoming a legal manufacturer when rebranding, the most effective strategy is a formal “Private Label” agreement with the original OEM.
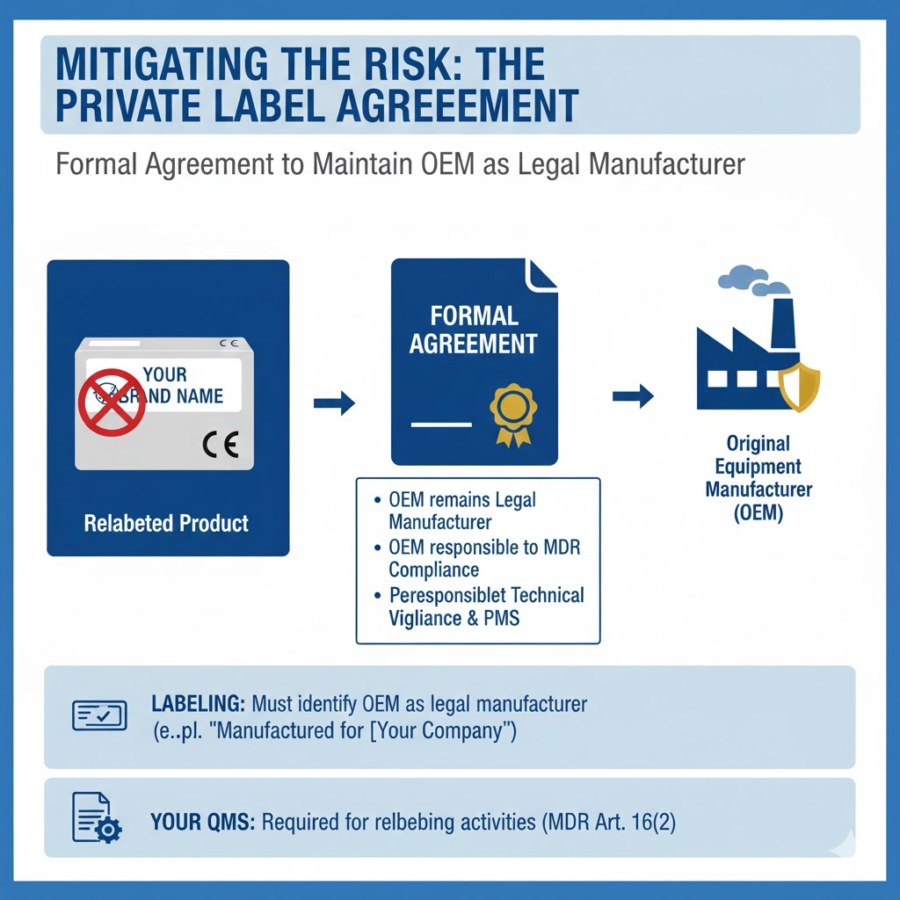
This agreement must explicitly state that:
-
The OEM remains the legal manufacturer.
-
The OEM is solely responsible for MDR/IVDR compliance, including CE marking, technical documentation, vigilance, and post-market surveillance.
Key considerations for this path:
-
Labeling: Even with an agreement, the device labeling must still identify the OEM as the legal manufacturer, often alongside your trade name (e.g., “Manufactured by [OEM Name] for [Your Company Name]”).
-
Your QMS: If you perform any relabeling, repackaging, or translating of instructions, you still need a Quality Management System (QMS) (e.g., ISO 13485) to ensure these activities do not compromise the device’s original condition, as per Article 16(2).
Conclusion
Understanding and proactively addressing Article 16 is crucial for any economic operator in the EU medical device supply chain. Unintentional manufacturer status carries significant regulatory, financial, and liability implications. By carefully structuring agreements and clearly defining responsibilities, companies can effectively market devices under their brand while ensuring full compliance with the EU MDR/IVDR.