Ancillary Medicinal Substance (AMS) and Regulation (EU) 2017/745
Under the EU Medical Device Regulation (EU MDR 2017/745), an Ancillary Medicinal Substance (AMS) refers to a substance that:
Forms an integral part of a medical device and, if used separately, would be considered a medicinal product, but whose action within the device is ancillary to the primary intended action of the medical device.
Key EU MDR Definition (Article 1(8))
-
The primary mode of action of the product must be that of a medical device (i.e., not pharmacological, immunological, or metabolic).
-
The medicinal substance supports the device function but does not provide the principal therapeutic effect.
Typical Examples under EU MDR
-
Drug-coated stents where the drug reduces restenosis but the stent’s mechanical action is primary
-
Antimicrobial coatings on catheters to reduce infection risk
-
Heparin-coated devices to improve blood compatibility
-
Devices containing anesthetic or anti-inflammatory substances with supportive action
MDR Regulatory-Safe Statement
Devices incorporating an ancillary medicinal substance and classified under Rule 14 of EU MDR 2017/745 must undergo verification of the quality, safety, and usefulness of the medicinal substance in accordance with the relevant methods set out in Annex I to Directive 2001/83/EC, as part of the conformity assessment procedure involving consultation with a competent authority for medicinal products.
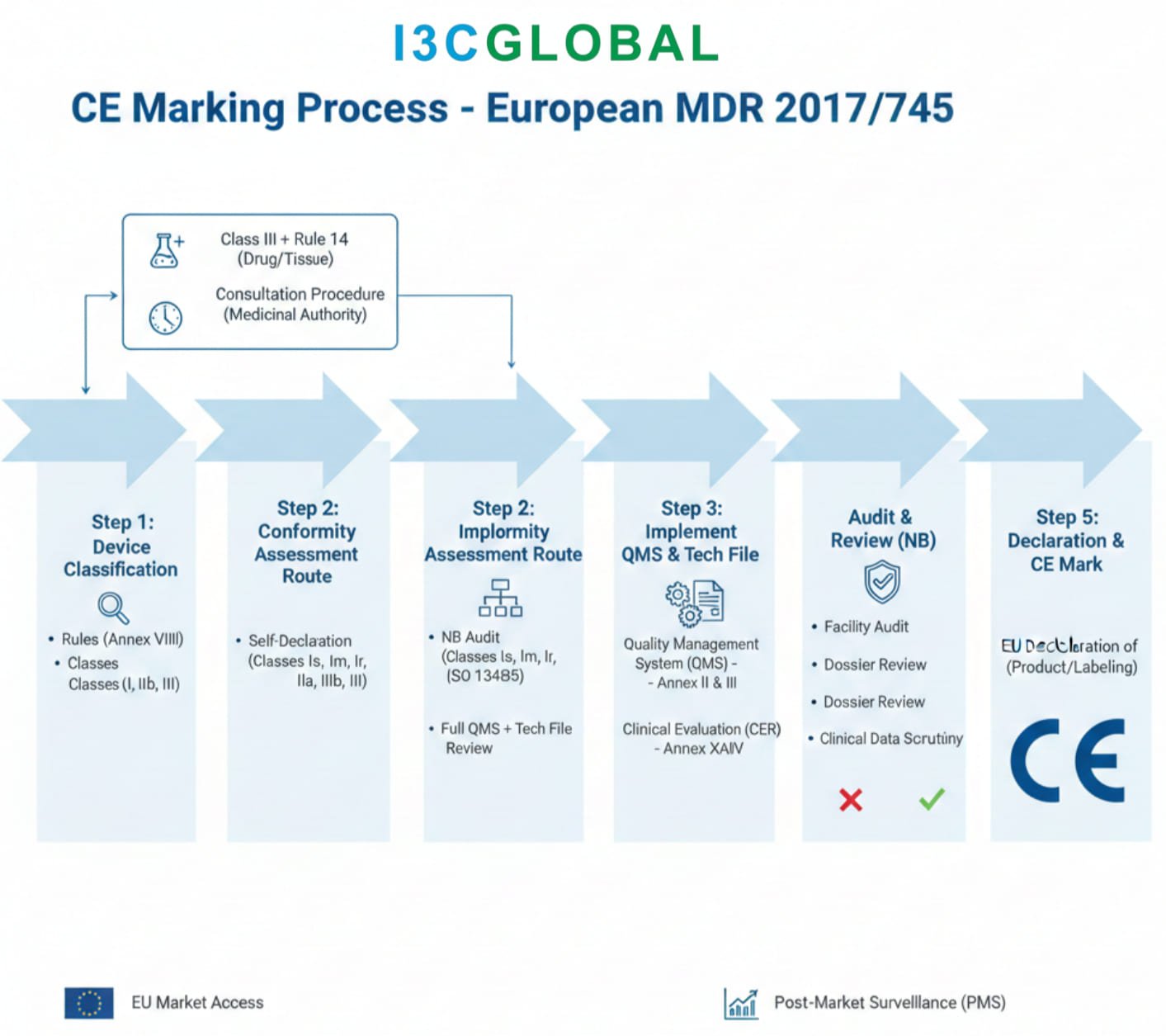
Dossier Requirements
In order to facilitate review, typically performed by a quality, a non-clinical and a clinical assessor; it is necessary to provide the dossier as separate, indexed and searchable (pdf) modules. Each module should be less than 100 MB to comply with Competent Authority requirements. If necessary supportive information can be provided in Appendices to the Modules. An index showing the folder structures and document titles should be provided. See reference section below for more detailed guidance, in particular the EMA dossier guidance.



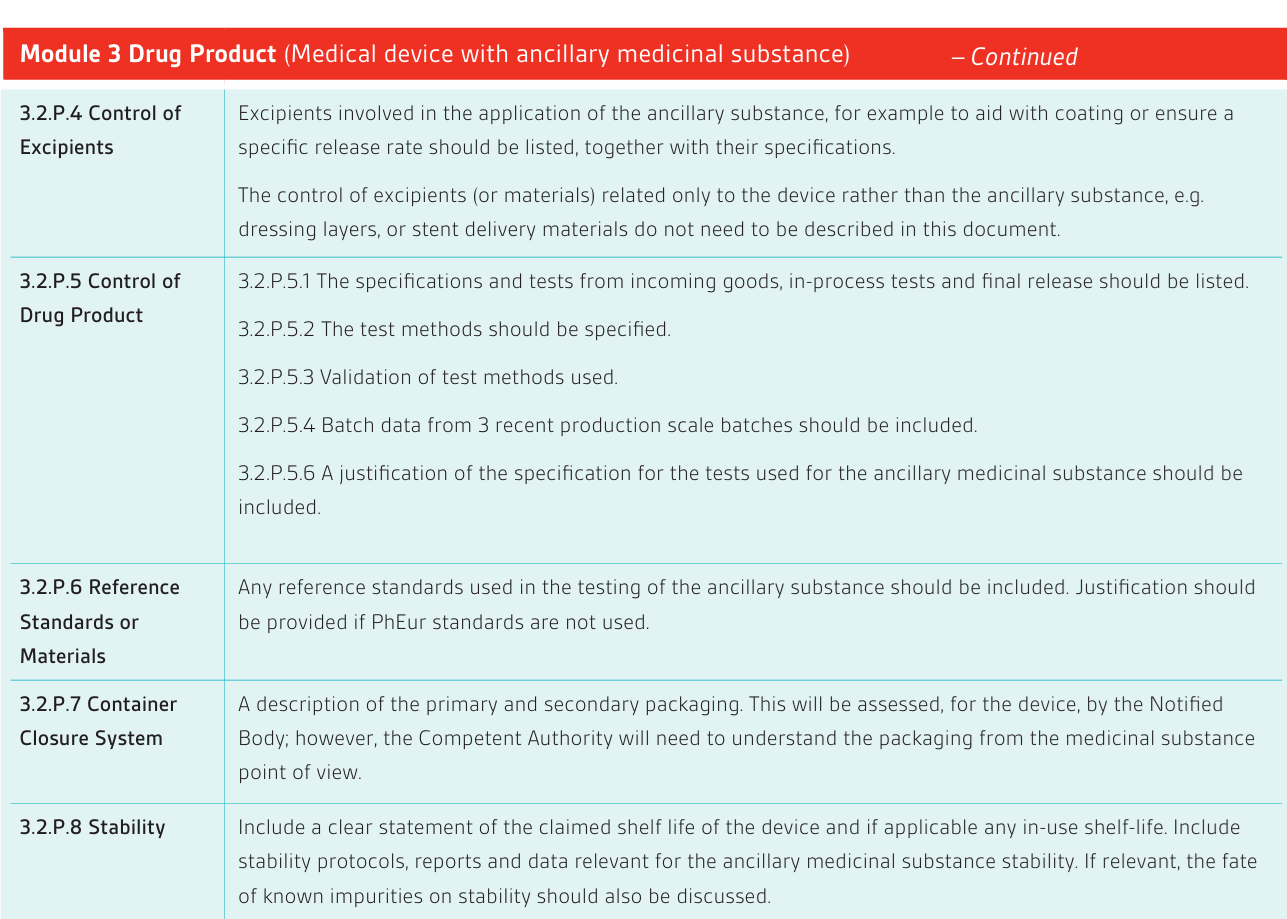

**Image and information sourced from BSI