Quick Contact
Just supply the infomation requested by us. We develop your Technical Documentation for you

What is Class III Medical Device?
The definition and compliance requirements for Class III Medical Device is more stringent than for all other classes, particularly with respect to demonstrating device quality, safety, and performance during the conformity assessment procedures required for placing them on the EU market. Some crucial information for manufacturers seeking to understand and comply with the regulatory framework for high-risk medical devices in Europe is provided below.
Importance of Class III Medical Device!
Class III Medical Device is the highest risk category of medical devices under the EU MDR 2017/745. These devices pose the greatest potential risk to the patient and/or user. They typically sustain or support life, are implanted in the body, or control critical bodily functions. Due to their high-risk nature, Class III devices are subject to the most rigorous regulatory scrutiny. A few examples of Class III include:
- Long term and short term Implantable devices
- Prosthetic valves (Heart)
- Active stimulators
- Bone replacements
Do you need an email containing full details within 2 minutes?
Key Requirements for Class III Medical Device under EU MDR
Under the EU MDR 2017/745, manufacturers of Class III Medical Device meet several strict regulatory requirements. These requirements focus on clearly demonstrating the safety and performance of high-risk devices for both patients and regulatory authorities. The key requirements include the following:
- Technical Documentation: Manufacturers must prepare detailed technical documentation file for their Class III Device. This file must contain adequate information to demonstrate that the device meets the relevant General Safety and Performance Requirements (GSPRs) outlined in Annex I of the EU MDR.
-
Clinical Evaluation: A thorough and detailed clinical evaluation is mandatory for Class III devices. Manufacturers must do literature search or conduct clinical investigations to gather sufficient clinical evidence to demonstrate the safety and performance of their devices in line with the intended purpose. The clinical evaluation must be based on an evaluation of relevant scientific literature, clinical experience, and clinical investigation results.
-
Quality Management System (QMS): Manufacturers must establish and maintain a robust medical device quality management system (MDQMS) that complies with the requirements of ISO 13485. The QMS should cover all aspects of the device lifecycle, from design and development to manufacturing, distribution, and post-market surveillance.
-
Unique Device Identification (UDI): The EU MDR mandates the use of a UDI system for medical devices and IVD,s. Manufacturers must assign a UDI barcode to their all scalable units of Class III medical device and submit the UDI information to the EUDAMED. The UDI system facilitates traceability and post-market surveillance.
-
Post-Market Surveillance (PMS): Manufacturers are required to implement a comprehensive PMS system to actively monitor the performance of their devices after they are placed on the market. (Post CE Certification)
-
Person Responsible for Regulatory Compliance (PRRC): Manufacturers must designate a PRRC who is responsible for ensuring that the device complies with the requirements of the EU MDR.
Conformity Assessment Procedures for Class III Medical Device
Class III Medical Device are subject to a rigorous conformity assessment procedure that involves a Notified Body. The Notified Body is an independent organization designated by a Member State to assess the conformity of medical devices with the requirements of the EU MDR. The conformity assessment procedure for Class III Devices typically involves the following steps as shown in the below image
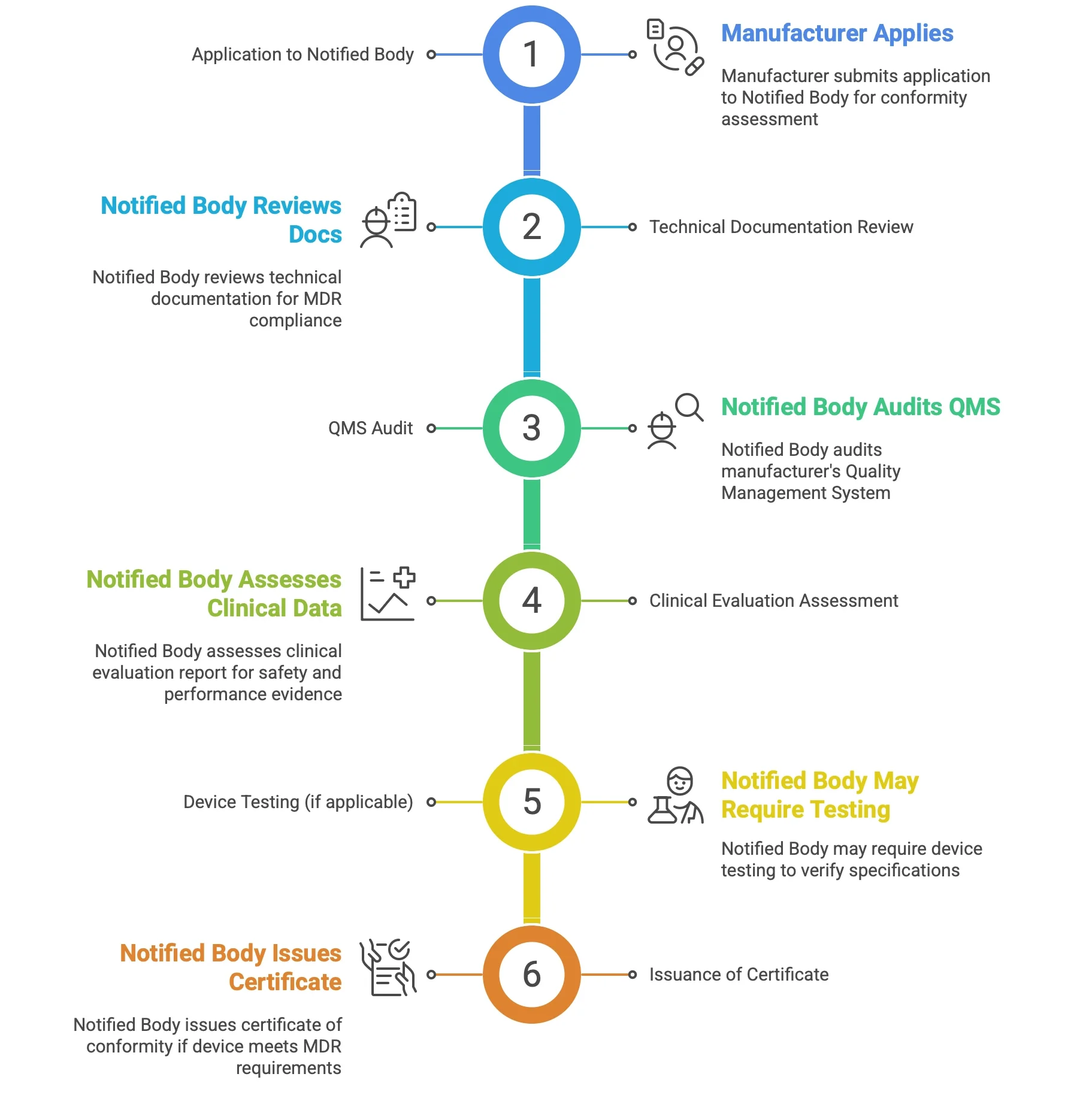
Class III Medical Device face the highest Notified Body scrutiny during technical documentation review, where delays, non-compliance, and weak clinical strategies can cost manufacturers in terms of resources and financially. Due to this issues demands for a strategic partner company like I3CGlobal with proven capability.
I3CGlobal empowers manufacturers by delivering end-to-end technical and scientific solutions from strategy planning, documentation, and Notified body conformity assessment support. With I3CGlobal, manufacturers accelerate CE Certification and EU Market entry.
Class III Medical Device Challenges
Navigating the regulatory landscape for Class III medical devices under the EU MDR can be challenging. Manufacturers need to:
-
Allocate sufficient resources to ensure compliance.
-
Develop a robust Quality management system.
-
Conduct thorough clinical evaluations.
-
Establish a comprehensive PMS system.
-
Stay up-to-date with the latest regulatory requirements.
-
Choose a Notified Body carefully.
Class III Medical Device Consultants in USA, India and UK
Class III Medical Device, regulatory success under EU MDR demands seasoned experts with proven experience in high-risk device compliance and clinical evaluation. I3CGlobal brings specialized Class III expertise to guide manufacturers through the most complex regulatory challenges, ensuring faster approvals, reduced risk, and sustained EU market access.
- Technical Documentation guidance & preparation
- Notified Body submission and answering to the review comments
- Guides in Biological Evaluation
- Review the external protocols and reports
- Prepares Clinical Evaluation Report as per Meddev 2.7/1 Rev 4
- Conducts Risk Analysis and prepares Risk Management File as per EN ISO 14971
- Arrange Notified Body and coordinate with them till the issue of the CE Certificate
- Arranges EU Representative from the European Union
- Arrange a Free Sale Certificate from the European Union
- Develops Post Market Surveillance plan, Post Market Clinical follow-up plan and report and Periodic Safety Update Report.
Frequently Asked Questions
Does Clinical investigations and equivalence shall be performed for Class III Medical Device CE Marking?
- If equivalent devices have sufficient clinical data and the manufacturer conducted PMCF studies, if the existing device is CE certified or you have access to the ongoing data of the equivalent device manufacturer
- If the devices have sufficient clinical data compliant with CS if available for devices such as Sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips or connectors
What are the devices covered in the new device reclassifications as per Annex VIII?
- Implantable contraceptives
- Absorbable non-implants (eg skin or GI)
- Total and partial joint replacement implants
- Implants in contact with the spinal column
- Devices incorporating nanomaterials
- Non-invasive devices used in direct contact with human cells for IVF
- Devices incorporating human-derived substances
- Software that could have an impact that may cause death or irreversible deterioration of a person’s state of health
